Abstract
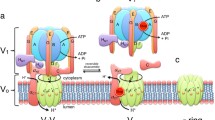
Our current work on a vacuolar membrane proton ATPase in the yeastSaccharomyces cerevisiae has revealed that it is a third type of H+-translocating ATPase in the organism. A three-subunit ATPase, which has been purified to near homogeneity from vacuolar membrane vesicles, shares with the native, membrane-bound enzyme common enzymological properties of substrate specificities and inhibitor sensitivities and are clearly distinct from two established types of proton ATPase, the mitochondrial F0F1-type ATP synthase and the plasma membrane E1E2-type H+-ATPase. The vacuolar membrane H+-ATPase is composed of three major subunits, subunita (M r =67 kDa),b (57kDa), andc (20 kDa). Subunita is the catalytic site and subunitc functions as a channel for proton translocation in the enzyme complex. The function of subunitb has not yet been identified. The functional molecular masses of the H+-ATPase under two kinetic conditions have been determined to be 0.9–1.1×105 daltons for single-cycle hydrolysis of ATP and 4.1–5.3×105 daltons for multicycle hydrolysis of ATP, respectively.N,N′-Dicyclohexylcarbodiimide does not inhibit the former reaction but strongly inhibits the latter reaction. The kinetics of single-cycle hydrolysis of ATP indicates the formation of an enzyme-ATP complex and subsequent hydrolysis of the bound ATP to ADP and Pi at a 7-chloro-4-nitrobenzo-2-oxa-1,3-diazolesensitive catalytic site. Cloning of structural genes for the three subunits of the H+-ATPase (VMA1, VMA2, andVMA3) and their nucleotide sequence determination have been accomplished, which provide greater advantages for molecular biological studies on the structure-function relationship and biogenesis of the enzyme complex. Bioenergetic aspects of the vacuole as a main, acidic compartment ensuring ionic homeostasis in the cytosol have been described.
Similar content being viewed by others
Abbreviations
- CCCP:
-
carbonyl cyanidem-chlorophenyl hydrazone
- DCCD:
-
N,N′-dicyclohexylcarbondiimide
- DES:
-
diethylstilbestrol
- DIDS:
-
4,4′-diisothiocyano-2,2′-stilbene disulfonic acid
- NBD-Cl:
-
7-chloro-4-nitrobenzo-2-oxa-1,3-diazole
- Pi:
-
inorganic phosphate
- SDS:
-
sodium dodecylsulfate
- SF6847:
-
3,5-di-tert-butyl-4-hydroxybenzylidenemalononitrile
- SITS:
-
4-acetamide-4′-isothiocyanatostilbene-2,2′-disulfonic acid
- ZW3-14:
-
N-tetradecyl-N,N′-dimethyl-3-ammonio-1-propanesulfonate
References
Al-Awquati, Q (1986)Annu. Rev. Cell Biol. 2, 179–199.
Anraku, Y. (1987a) InBioenergetics: Structure and Function of Energy Transducing Systems (Ozawa, T., and Papa, S., eds.), Japan Scientific Societies Press and Academic Press, Tokyo and New York, pp. 249–262.
Anraku, Y. (1987b) InPlant Vacuoles (Marin B., ed.), Plenum Press, New York and London, pp. 255–265.
Anraku, Y., Uchida, E., and Ohsumi, Y. (1987) InPerspectives of Biological Energy Transduction (Mukohata, Y., Morales, M. F., and Fleischer, S., eds.), Academic Press, Yokyo and New York, pp. 309–313.
Bowman, B. J., and Bowman, E. J. (1986)J. Membr. Biol. 94, 83–97.
Bowman, B. J., Berenski, C. J., and Jung, C. Y. (1985)J. Biol. Chem. 260, 8726–8730.
Bowman, E. J., Mandala, S., Taiz, L., and Bowman, B. J. (1986)Proc. Natl. Acad. Sci. USA 83, 48–52.
Bowman, B. J., Allen, R., Wechser, M. A., and Bowman, E. J. (1988a)J. Biol. Chem. 263, 14002–14007.
Bowman, E. J., Tenney, K., and Bowman, B. J. (1988b)J. Biol. Chem. 263, 13994–14001.
Cross, R. L., Grubmeyer, C., Penefsky, H. S. (1982)J. Biol. Chem. 257, 12101–12105.
Denda, K., Konishi, J., Oshima, T., Date, T., and Yoshida, M. (1988)J. Biol. Chem. 263, 6012–6015.
De Vries, H. (1985)Jahrb. Wiss. Bot. 16, 465–598.
Duncan, T. M., and Senior, A. E. (1985)J. Biol. Chem. 260, 4901–4907.
Futai, M., Noumi, T., and Maeda, M. (1988)J. Bioenerg. Biomembr. 20, 41–58.
Grubmeyer, C., Cross, R. L., and Penefsky, H. S. (1982)J. Biol. Chem. 257, 12092–12100.
Hirata, R., Ohsumi, Y., and Anraku, Y. (1989)FEBS Lett. 244, 397–401.
Kaestner, K. H., Randall, S. K., and Sze, H. (1988)J. Biol. Chem. 263, 1282–1287.
Kakinuma, Y., Ohsumi, Y., and Anraku, Y. (1981)J. Biol. Chem. 256, 10859–10863.
Kitamoto, K., Yoshizawa, K., Ohsumi, Y., and Anraku, Y. (1988a)J. Bacteriol. 170, 2683–2686.
Kitamoto, K., Yoshizawa, K., Ohsumi, Y., and Anraku, Y. (1988b)J. Bacteriol. 170, 2687–2691.
Lai, S., Randall, S. K., and Sze, H. (1988)J. Biol. Chem. 263, 16731–16737.
Lichke, L. P., and Okorokov, L. A. (1985)FEBS Lett. 187, 349–352.
Mandala, S., and Taiz, L. (1985)Plant Physiol. 78, 327–333.
Mandel, M., Moriyama, Y., Hulmes, J. D., Pan, Y.-C. E., Nelson, H., and Nelson, N. (1988)Proc. Natl. Acad. Sci. USA 85, 5521–5524.
Manolson, M. F., Rea, P. A., and Poole, R. J. (1985)J. Biol. Chem. 260, 12273–12279.
Manolson, M. F., Percy, J. M., Apps, D. K., Xie, X. S., Stone, D. K., and Poole, R. J. (1987) InProceedings of the Membrane Protein Symposium (Goheen, S. C., ed.), Bio-Rad, Richmond, California, pp. 427–434.
Manolson, M. F., Ouellette, B. F. F., Filion, M., and Poole, R. J. (1988)J. Biol. Chem. 263, 17987–17994.
Marin, B. P., Preisser, J., and Komor, E. (1985)Eur. J. Biochem. 151, 131–140.
Matile, P. (1975)The Lytic Compartment of Plant Cells, Springer-Verlag, Wien and New York, pp. 1–175.
Mellman, I., Fuchs, R., and Helenius, A. (1986)Annu. Rev. Biochem. 55, 663–700.
Nelson, N. (1988)Plant Physiol. 86, 1–3.
Nelson, H., Mandiyan, S., and Nelson, N. (1989)J. Biol. Chem. 264, 1775–1778.
Noumi, T., Taniai, M., Kanazawa, H., and Futai, M. (1986)J. Biol. Chem. 261, 9196–9201.
Ohkuma, S. (1987) InLysosomes: Their Role in Protein Breakdown (Glaumann, H., and Ballard, F. J., eds.), Academic Press, Newe York, pp. 115–148.
Ohsumi, Y., and Anraku, Y. (1981)J. Biol. Chem. 256, 2079–2082.
Ohsumi, Y., and Anraku, Y. (1983)J. Biol. Chem. 258, 5614–5617.
Ohsumi, Y., Kitamoto, K., and Anraku, Y. (1988)J. Bacteriol. 170, 2676–2682.
Randall, S. K., and Sze, H. (1987)J. Biol. Chem. 262, 7135–7141.
Rudnick, G. (1986)Annu. Rev. Physiol. 48, 403–413.
Rea, P. A., Griffith, C. J., and Sanders, D. (1988)J. Biol. Chem. 263, 14745–14752.
Sato, T., Ohsumi, Y., and Anraku, Y. (1984a)J. Biol. Chem. 259, 11505–11508.
Sato, T., Ohsumi, Y., and Anraku, Y. (1984b)J. Biol. Chem. 259, 11509–11511.
Sze, H. (1985)Annu. Rev. Plant Physiol. 36, 175–208.
Tanifuji, M., Sato, M., Wada, Y., Anraku, Y., and Kasai, M. (1988)J. Membr. Biol. 106, 47–55.
Uchida, E., Ohsumi, Y., and Anraku, Y. (1985)J. Biol. Chem. 260, 1090–1095.
Uchida, E., Ohsumi, Y., and Anraku, Y. (1988a)J. Biol. Chem. 263, 45–51.
Uchida, E., Ohsumi, Y., and Anraku, Y. (1988b) InMethods in Enzymology (Biomembrane, Part Q) (Fleischer, S., and Fleischer, B., eds.), Vol. 157, Academic Press, New York, pp. 544–561.
Wada, Y., Ohsumi, Y., and Anraku, Y. (1986)Cell Struct. Funct. 11, 533.
Wada, Y., Ohsumi, Y., Tanifuji, M., Kasai, M., and Anraku, Y. (1987)J. Biol. Chem. 262, 17260–17263.
Yoshihisa, T., Ohsumi, Y., and Anraku, Y. (1988)J. Biol. Chem. 263, 5158–5163.
Zimniak, L., Dittrich, P., Gogarten, J. P., Kibak, H., and Taiz, L. (1988)J. Biol. Chem. 263, 9102–9112.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anraku, Y., Umemoto, N., Hirata, R. et al. Structure and function of the yeast vacuolar membrane proton ATPase. J Bioenerg Biomembr 21, 589–603 (1989). https://doi.org/10.1007/BF00808115
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00808115