Abstract
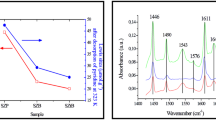
Ultraviolet Raman spectroscopy (UVRS) has been demonstrated to be a powerful new tool for catalysis and surface science studies. UVRS can successfully avoid the surface fluorescence which frequently occurs in normal Raman spectra of many catalysts. Fresh, deactivated and regenerated sulfated-zirconia catalysts have been characterized using this new method. The UV Raman spectrum of the fresh sample is dominated by the tetragonal phase, however, the spectrum of deactivated sulfated zirconia is nearly identical to that of the monoclinic phase of pure zirconia. A regeneration of the deactivated catalyst restores the spectrum back to that of the fresh sample. In addition, a new band at 750 cm−1 associated with the fresh sample attenuates with the deactivation process. This band is tentatively assigned to the surface [ZrO4]4− unit which is assumed to bond to the surface sulfate group. The results indicate that the surface phase of sulfated zirconia is reconstructed from tetragonal to monoclinic phase during the deactivation process while the bulk remains in the tetragonal phase after the deactivation. It is proposed that the surface tetragonal phase is stabilized by sulfate groups, and is associated with the catalytic activity.
Similar content being viewed by others
References
J.M. Stencel,Raman Spectroscopy for Catalysis (Van Nostrand Reinhold, New York, 1990).
A. Campion, in:Vibrational Spectroscopy of Molecules on Surfaces, eds. J.T. Yates Jr. and T.E. Madey (Plenum Press, New York, 1987) p. 345.
C.R. Johnson and S.A. Asher, Anal. Chem. 56 (1984) 2261.
S.A. Asher, Anal. Chem. 65 (1993) 201A.
R.W. Bormett and S.A. Asher, J. Appl. Phys. 77 (1995) 5916.
S.A. Asher, Anal. Chem. 65 (1993) 57A.
T. Yamaguchi, T. Jin, T. Ishida and K. Tanabe, Mater. Chem. Phys. 17 (1987) 3.
M. Waqif, J. Bachelier, O. Saur and J.C. Lavalley, J. Mol. Catal. 72 (1992) 127.
C. Morterra, G. Cerrato, C. Emanuel and V. Bolis, J. Catal. 142 (1993) 349.
V. Adeeva, J.W. de Haan, J. Janchen, G.D. Lei, V. Schunemann, L.J.M. van de Ven, W.M.H. Sachtler and R.A. van Santen, J. Catal. 151 (1995) 364.
D.A. Ward and E.I. Ko, J. Catal. 150 (1994) 18.
R. Srinivasan, R.A. Keogh, D.R. Milburn and B.H. Davis, J. Catal. 153 (1995) 123.
F.R. Chen, G. Coudurier, J.-F. Joly and J.C. Vedrine, J. Catal. 143 (1993) 616.
T. Riemer, D. Spidbauer, M. Hunger, G.A.H. Mekhemer and H. Knözinger, J. Chem. Soc. Chem. Commun. (1994) 1181.
C. Morterra, G. Cerrato, F. Pinnar and M. Signoretto, J. Phys. Chem. 98 (1994) 12373.
L.M. Kustov, V.B. Kazansky, F. Figueras and D. Tichit, J. Catal. 150 (1994) 143.
J.R. Sohn and H.J. Jany, J. Mol. Catal. 64 (1991) 349.
E. Escalona Platero and M. Peñarroya Mentruit, Catal. Lett. 30 (1995) 31.
G.H. Smudde Jr. and P.C. Stairz, Surf. Sci. 317 (1994) 65.
R.A. Comelli, C.R. Vera and J.M. Parera, J. Catal. 151 (1995) 96.
P.D.L. Mercera, J.G. van Ommen, E.B.M. Doesburg, A.J. Burggraaf and J.R.H. Ross, Appl. Catal. 57 (1990) 127.
F. Gonzalez-Vilehez and W.P. Griffith, J. Chem. Soc. Dalton Trans. (1972) 1416.
R. Srinivasan, D. Taulbee and B.H. Davis, Catal. Lett. 9 (1991) 1.
J.R. Sohn and H.W. Kim, J. Mol. Catal. 52 (1989) 361.
T. Ishida, T. Yamaguchi and K. Tanabe, Chem. Lett. (1988) 1869.
M. Bensitel, O. Saur, J.C. Lavalley and B.A. Morrow, Mater. Chem. Phys. 19 (1988) 147.
Author information
Authors and Affiliations
Additional information
Visiting scholar on leave from State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, PR China.
Rights and permissions
About this article
Cite this article
Li, C., Stair, P.C. Ultraviolet Raman spectroscopy characterization of sulfated zirconia catalysts: fresh, deactivated and regenerated. Catal Lett 36, 119–123 (1996). https://doi.org/10.1007/BF00807606
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00807606