Summary
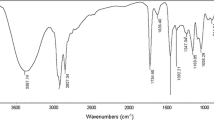
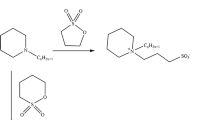
N-Octyloxycarbonylmethyl pyridinium bromide (C 8), N-dodecyloxycarbonylmethyl pyridinium bromide (C 12) and N-cetyloxycarbonylmethyl pyridinium bromide (C 16) were prepared. Several studies were carried out with their aqueous solutions. Surface tensions, electrical conductivities, and biodegradabilities were evaluated. The antibacterial and antifungal activities of the cationic surfactants were studied. Surface properties, particularly critical micelle concentration (CMC), effectiveness (Π CMC ), efficiency (P c20), maximum surface excess (Γmax), and minimum surface area (A min) were investigated at different concentrations at 20, 35, and 50°C respectively. Free energies, enthalpies, entropies of micellization, and adsorption of the surfactants in the aqueous solution were studied.
Zusammenfassung
Verschiedene Untersuchungen an wäßrigen Lösungen von N-Octyloxycarbonylmethyl-pyridiniumbromid (C 8), N-Dodecyloxycarbonylmethyl-pyridiniumbromid (C 12) und N-Cetyloxycarbonylmethyl-pyridiniumbromid (C 16) wurden durchgeführt. Oberflächenspannungen, elektrische Leitfähigkeiten und biologische Abbaubarkeit wurden bestimmt. Die bakterizide und fungizide Aktivität der kationischen Tenside wurde untersucht. Oberflächenparameter, insbesondere kritische Micellenkonzentration (CMC), Effektivität (ΠCMC), EffizienzP c20), maximaler Oberflächenüberschuß (Γmax) und minimale Oberfläche (A min) wurden bei verschiedenen Konzentrationen und bei Temperaturen von 20, 35 und 50°C untersucht. Die freien Energien, Enthalpien, Entropien und die Asorption der Tenside in wäßriger Lösung wurden bestimmt.
Similar content being viewed by others
References
US Pat. 2,023,075
US Pat. 2,190.133
US Pat. 2.213.979
US Pat. 2,317,378
US Pat. 2,321,594
Koshy L, Pegiadou KS, Rakshit AK (1991) Colloids Surf59: 1
Fisicaro E, Pelizzetti E, Barbieri M, Savarino P, Viscardi G (1990) Thermochim Acta168: 143
Rosen MJ, Cohen AW, Dahanayake M, Hua XY (1982) J Phys Chem86: 541
Dahanayake M, Cohen AW, Rosen MJ (1986) J Phys Chem90: 2413
Gad EAM, El-Sukkary MMA, Ismail DA (1977) Am Oil Chem Soc74: 43
Hikota T, Morohara K, Meguro K (1970) Bull Chem Soc Japan43: 3913
Rosen MJ (1978) Surfactants and interfacial phenomena. Wiley, New York, p 61
Takeshi H (1970) Bull Chem Soc Japan43: 2236
Throkmorton PE, Egan RR, Aelony D (1974) J Oil Chem Soc51; 486
Abo Zeid AA, Shehata YM (1969) Indian J Pharm31(3): 72
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gad, E.A.M., El-Sukkary, M.M.A. & Azzam, E.M.S. Surface and thermodynamic studies of N-((octyl, dodecyl, and cetyl) oxycarbonylmethyl) pyridinium bromide. Monatsh Chem 128, 1237–1246 (1997). https://doi.org/10.1007/BF00807255
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00807255