Summary
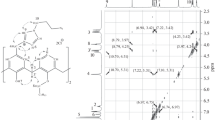
New macrotricyclic compounds consisting of two calix[4]arene substructures connected by aliphatic chains of various length (three to five carbon atoms) between two oppositep-positions and two distal phenolic oxygens have been synthesized. Starting withp-tert-butyl-calix[4]arene, two O-protected phenolic units are attachedvia ether links in 1,3-position by reaction with the corresponding tosylates. After deprotection, the new calix[4]arene is formed by fragment condensation with 2,6-bisbromomethylated 4-alkylphenols. The structure of one example (8c) has been confirmed by single crystal X-ray analysis. Both calixarene parts assume the cone conformation, a molecule of acetonitrile being included in both cavities.
Zusammenfassung
Neue makrotricyclische Verbindungen, in denen zwei Calix[4]aren-Einheiten durch aliphatische Ketten unterschiedlicher Länge (drei bis fünf C-Atome) zwischen zwei gegenüberliegendenp-Positionen und zwei gegenüberliegenden Phenolsauerstoffen verknüpft sind, wurden hergestellt. Ausgehend vonp-tert-Butyl-calix[4]aren werden zunächst zwei O-geschützte Phenolbausteine in 1,3-Stellung durch Umsetzung mit den entsprechenden Tosylaten etherartig gebunden. Nach Abspaltung der Benzylether-Schutzgruppe wird durch Fragmentkondensation mit 2,6-bisbrommethylierten 4-Alkylphenolen das neue Calix[4]aren gebildet. Für8c wurde die Struktur durch Einkristallröntgenstrukturanalyse bestätigt. Beide Calixarenteile nehmen diecone-Konformation ein, wobei in die beiden Hohlräume je ein Molekül Acetonitril eingeschlossen wird.
Similar content being viewed by others
References
Böhmer V (1995) Angew Chem107: 785; (1995) Angew Chem Int Ed Engl34: 713
Koh K, Araki K, Shinkai S (1994) Tetrahedron Lett35: 8255
Shimizu KD, Rebek Jr J (1995) J Proc Nat Acad Sci92: 12403;
Mogck O, Böhmer V, Vogt W (1996) Tetrahedron52: 8489;
Hamann BC, Shimizu KD, Rebek Jr J (1996) Angew Chem108: 1425 (1996) Angew Chem Int Ed Engl35: 1326;
Mogck O, Paulus EF, Böhmer V, Thondors I, Vogt W (1996) Chem Commun 2533
Chapman RG, Sherman J C (1995) J Am Chem Soc117: 9081
Byun Y-S, Vadhat O, Blanda MT, Knobler CB, Cram DJ (1995) J Chem Soc Chem Commun 1825;
Byun Y-S, Robbins TA, Knobler CB, Cram DJ (1995) ibid 1947;
Eid Jr CN, Knobler CB, Gronbeck DA, Cram DJ (1994) J Am Chem Soc116: 8506;
Robbins TA, Knobler CB, Bellew DR, Cram DJ (1994) ibid116: 111
Timmerman P, Verboom W, van Veggel FCJM, van Duynhoven JPM, Reinhoudt DN (1994) Angew Chem106: 2437; (1994) Angew Chem Int Ed Engl33: 2345
Fraser JR, Borecka B, Trotter J, Sherman JC (1995) J Org Chem60: 1207
Blanda MT, Griswold KE (1994) J Org Chem59: 4313;
Araki K, Sisido K, Hisaichi K, Shinkai S (1993) Tetrahedron Lett34: 8297
Böhmer V, Goldmann H, Vogt W, Vicens J, Asfari Z (1989) Tetrahedron Lett30: 1391
Hajek F, Graf E, Hosseini MW (1966) Tetrahedron Lett37: 1409
Kraft D, van Loon J-D, Owens M, Verboom W, Vogt W, McKervey MA, Böhmer V, Reinhoudt DN (1990) Tetrahedron Lett31: 4941;
Beer PD, Keefe AD, Slawin AMZ, Williams DJ (1990) J Chem Soc Dalton Trans 3675
Lhotak P, Shinkai S (1995) Tetrahedron51: 7861;
Mogck O, Böhmer V, Vogt W (unpublished results)
Ikeda A, Shinkai S (1994) J Chem Soc Chem Commun 2375;
Rérez-Adelmar J-A, Abraham H, Sánchez C, Rissanen K, Prados P, de Mendoza J (1996) Angew Chem108: 1088; (1996) Angew Chem Int Ed Engl35: 1009
Ross H, Lüning U (1995) Angew Chem107: 2723; (1995) Angew Chem Int Ed Engl34: 2555;
Zhong ZL, Chen Y-Y, Lu XR (1995) Tetrahedron Lett36: 6735;
Ohseto F, Shinkai S (1995) J Chem Soc Perkin Trans 2, 1103
Arduini A, Fanni S, Manfredi G, Pochini A, Ungaro R, Sicuri AR, Ugozzoli F (1995) J Org Chem60: 1448;
Fujimoto K, Shinkai S (1994) Tetrahedron Lett35: 2915;
Arduini A, Pochini A, Sicuri AR, Secchi A, Ungaro R (1994) Gazz Chim Ital124: 129
Wasikiewicz W, Rokicki G, Kiełkiewicz J, Böhmer V (1994) Angew Chem106: 230; (1994) Angew Chem Int Ed Engl33: 214
Beer PD, Gale PA, Hesek D (1995) Tetrahedron Lett36: 767
Goldmann H, Vogt W, Paulus E, Böhmer V (1988) J Am Chem Soc110: 6811
Olmstead MM, Sigel G, Hope H, Xu X, Power PP (1985) J Am Chem Soc107: 8087;
Bott SG, Coleman AW, Atwood JL (1986) J Chem Soc Chem Commun 610;
Andreetti GD, Calestani G, Ugozzoli F, Arduini A, Ghidini E, Pochini A, Ungaro R (1987) J Incl Phenom5: 123
Sternhel S (1969) Quart Rev23: 236
Groenen LC, Brunink J AJ, Bakker WI, Harkema S, Wijmenga SS, Reinhoudt DN (1992) J Chem Soc Perkin Trans 2, 1899
Perrin M, Oehler D (1990) In: Vicens J, Böhmer V (eds) Calixarenes, a Versatile Class of Macrocyclic Compounds. Kluwer, Dordrecht, pp 65–85;
Andreetti GD, Ugozzoli F (1990) In: ibid, pp 87–123
McKervey MA, Seward EM, Ferguson G, Ruhl BL (1986) J Org Chem51: 3581;
Xu W, Puddephatt RJ, Manojlovic-Muir L, Muir KW, Frampton CS (1994) J Incl Phenom Mol Recogn19: 277;
Böhmer V, Dörrenbächer R, Frings M, Heydenreich M, de Paoli D, Vogt W, Ferguson G, Thondorf I (1996) J Org Chem61: 549
Hanley-Smith P, Whiting A D (1980) J Chem Soc Perkin Trans 1, 614
Martin EL (1936) J Am Chem Soc58: 1438
Kharasch N, Kalfayan SH (1956) J Org Chem21: 929
Gutsche CD, Iqbal M, Stewart D (1986) J Org Chem51: 742
Doherty DG (1955) J Am Chem Soc77: 4887
Sheldrick GM (1990) Acta CrystA46: 467
Sheldrick GM (1993) SHELXL-93 program for crystal structure refinement. University of Göttingen, Germany
Sheldrick GM (1986) SHELXS-86 program for the solution of crystal structures. University of Göttingen, Germany
Sheldrick GM (1991) SHELXTL-PLUS, an integrated system for solving, refining and displaying crystal structures from diffracting data. Siemens Analytical X-Ray Instruments Inc, Madison, Wisconsin, USA
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wąsikiewicz, W., Rokicki, G., Kiełkiewicz, J. et al. Head-to-tail connected double calix[4]arenes. Monatsh Chem 128, 863–879 (1997). https://doi.org/10.1007/BF00807096
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00807096