Abstract
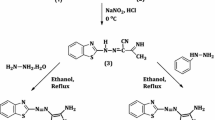
4,5-Dichloro-2-dicyanomethylene-4-cyclopenten-1,3-dione (2) is synthesized by partial Retro-Michael addition of tetracyanoethylene to 4,5-dichloro-4-cyclopentene-1,3-dione. Compound2 is a new electron acceptor, withN-methyl-benzthiazolone-2-hydrazone giving a charge-transfer complex (6). Nucleophilic substitution of2 by pyrrolidine, morpholine and piperidine leads to deeply coloured bisamides7,8. With aromatic amines bisamides are formed too (11), but monosubstitution products can be isolated.N,N-Dimethylaniline reacts with2 by elimination of hydrogen chloride, leading to aN,N-dimethylaminophenyl monosubstitution product of2. The bisamides are green-blue compounds with intense violet colour in solution. The dyes can be classified as pseudooxo croconic acid bisamides, the new type of chromophore is discussed by13C-spectroscopy and quantum chemical calculations (CNDO-CI).
Similar content being viewed by others
Literatur
West R., Niu J., in: Nonbenzoid Aromatics (Snyder J. P., Hrsg.). New York: Academic Press. 1969.
Fatiadi A. J., J. Amer. Chem. Soc.100, 2586 (1978); J. Org. Chem.45, 1338 (1980); Synthesis1978, 165.
Seitz G., Nachr. Chem. Tech. Lab.28, 804 (1980).
Zincke Th., Rohde A., Liebigs Ann. Chem.299, 377 (1898).
Roedig A., Hörnig L., Chem. Ber.88, 2003 (1955).
McBee E. T., Roberts C. W., Dinbergs K., J. Amer. Chem. Soc.78, 489 (1956).
Seitz G., Braun H., Arch. Pharm.309, 34 (1976).
Roedig A., Ziegler H., Chem. Ber.94, 1800 (1961).
Grohe K., Kaspers H., Scheinpflug H., Bayer AG, Ger. Offen. 2140737 (1973), Chem. Abstr.78, 124250 (1973);Grohe K., Frohberger P. E., Scheinpflug H., Bayer AG, Ger. Offen. 2248819 (1974), Chem. Abstr.81, 25186 (1974).
Kawada H., Hayashi S., Kasugai A., Shigematsu T., Japan Pat. 7779022 (1977), Chem. Abstr.88, 1597 (1978).
Wolfbeis O. S., Monatsh. Chem.112, 369 (1981).
Shigematsu T., Zomita M., Shibahara T., Inoue K., Japan Pat. 7783626 (1977), Chem. Abstr.88, 6414 (1978).
Iwataki I., Shibuya M., Nakata A., Mizuno M., Japan Pat. 78111039 (1978), Chem. Abstr.90, 54645 (1979); Japan Pat. 78101336 (1978), Chem. Abstr.90, 86852 (1979).
Fenske D., Becher H. J., Chem. Ber.108, 2115 (1975).
Junek H.,Sterk H., Tetrahedron Lett.1968, 4309.
Chatterjee S., Science157, 314 (1967).
Hünig S., Quast H., Liebigs Ann. Chem.711, 139 (1968).
Wheland R. C., Gillson J. L., J. Amer. Chem. Soc.98, 3916 (1976).
Barlow W. A., Davies G. R., Goodings E. P., Hand R. L., Rhodes M., Mol. Crist. Liq. Cryst.33, 123 (1976).
Junek H., Hermetter A., Fischer-Colbrie H., Wittmer-Metz M., Braun A. M., Chem. Ber.109, 1787 (1976).
Seitz G., Klein W., Arch. Pharm.305, 683 (1972).
Aigner H., Junek H., Sterk H., Monatsh. Chem.101, 1145 (1970);Junek H., Aigner H., Fischer-Colbrie H., Monatsh. Chem.103, 639 (1972);Fischer-Colbrie H., Aigner H., Junek H., Monatsh. Chem.106, 743 (1975);Junek H., Fischer-Colbrie H., Sterk H., Chem. Ber.110, 2276 (1977).
Junek H.,Hermetter A.,Fischer-Colbrie H.,Aigner H., Tetrahedron Lett.1973, 2939.
Junek H., Fischer-Colbrie H., Hermetter A., Z. Naturforsch.32, 898 (1977).
Rys P., Zollinger H., Leitfaden der Farbstoffchemie. Weinheim: Verlag Chemie. 1976.
Knothe L.,Prinzbach H.,Fritz H., Liebigs Ann. Chem.1977, 687.
Author information
Authors and Affiliations
Additional information
Herrn Prof. Dr.E. Ziegler zum 70. Geburtstag gewidmet.
Rights and permissions
About this article
Cite this article
Junek, H., Zuschnig, G., Thierrichter, R. et al. Tieffarbige Pseudooxo-Krokonsäurebisamide. Monatsh Chem 113, 1045–1058 (1982). https://doi.org/10.1007/BF00799246
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00799246