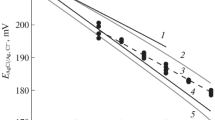
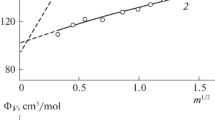
Abstract
The standard potentials of the silver, silver bromide electrode have been determined in 1,2-dimethoxyethane (DME) and in nineteenDME + water solvents from the e.m.f. measurements of cells of the type Pt|H2(g, 1 atm)|HBr (m), solvent|AgBr|Ag at intervals of 5°C from 5 to 45°C. The molality of HBr covered the range from 0.01 to 0.1 mol kg−1. In solvents of highDME content, where the dielectric constant is small, it was necessary to correct for ion-pair formation. The temperature variation of the standard potential has been used to evaluate the standard thermodynamic functions for the cell reaction, and the standard quantities for the transfer of HBr from water to the respective solvents. The results have been discussed both in relation to the acid-base nature of the solvent mixtures and also their structural effects on the transfer process.
Zusammenfassung
Die Standardpotentiale der Silber, Silberbromid-Elektrode wurden in 1,2-Dimethoxyethan (DME) und in 19 verschiedenenDME—Wasser-Gemischen aus EMK-Messungen der Zelle Pt|H2(g,1 atm)|HBr (m), Lsgsm.|AgBr|Ag in Temperaturintervallen von 5°C zwischen 5 und 45°C bestimmt. Die Molalität von HBr deckte den Bereich von 0,01 bis 0,1 mol kg−1. Bei Lösungen mit höheremDME-Gehalt — und damit niedrigen Dielektrizitätskonstanten —war es nötig, für die Bildung von Ionenpaaren eine Korrektur einzuführen. Über die Temperaturvariation wurden die thermodynamischen Größen für die Zellenreaktion und die Standardgrößen für den Transfer von HBr aus Wasser in das jeweilige Lösungsmittel bestimmt. Die Ergebnisse werden sowohl im Zusammenhang zur Säure-Base-Natur de Lösungsmittelmischungen als auch in bezug auf strukturelle Effekte im Transferprozeß diskutiert.
Similar content being viewed by others
References
Bates R. G., Hydrogen Bonded Solvent Systems (Covington A. K., Jones P., eds.). London: Taylor and Francis. 1968.
Bates R. G., Solute-Solvent Interactions (Coetzee J. F., Ritchie C. D., eds.), chapter 2. New York: Marcel Dekker. 1969.
Popovych O., Crit. Rev. Anal. Chem.1, 1 (1970).
Elsemongy M. M., Kenawy I. M., Fouda A. S., J. Chem. Soc., Faraday Trans.1, 78, 897 (1982).
Roy R. N., Robinson R. A., Bates R. G., J. Chem. Thermodynamics5, 559 (1973).
Roy R. N., Robinson R. A., Bates R. G., J. Amer. Chem. Soc.95, 8231 (1973).
Roy R. N., Swensson E. E., LaCross G., Krueger C. W., Adv. Chem. Ser.155, 220 (1976).
Elsemongy M. M., Fouda A., Amira M. F., Electrochim. Acta26, 255 (1981).
Elsemongy M. M., Fouda A. S., J. Chem. Soc., Faraday Trans.1, 77, 1169 (1981).
Elsemongy M. M., Fouda A. S., J. Chem. Thermodynamics13, 725 (1981).
Wallace W. J., Mathews A. L., J. Chem. Eng. Data8, 496 (1963).
Johnson D. A., Sen B., J. Chem. Eng. Data13, 376 (1968).
Mussini T., Formaro C. M., Andrigo P., J. Electroanal. Chem.33, 177 (1971).
Harned H. S., Owen B. B., The Physical Chemistry of Electrolytic Solutions, p. 459. New York: Reinhold. 1958.
Khoo K. H., J. Chem. Eng. Data17, 82 (1972).
Robinson R. A., Stokes R. H., Electrolyte Solutions, p. 353. London: Butterworths. 1965.
Bose K., Das K., Kundu K. K., J. Chem. Soc., Faraday Trans.1, 74, 1051. (1978).
Khoo K. H., Chan C., Aust. J. Chem.28, 721 (1975).
Das B. K., Das P. K., J. Chem. Soc., Faraday Trans.1, 74, 22 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Elsemongy, M.M., Kenawy, I.M. Standard potentials of the silver, silver bromide electrode and the thermodynamic properties of hydrobromic acid in 1,2-dimethoxyethane and its aqueous mixtures. Monatsh Chem 113, 877–886 (1982). https://doi.org/10.1007/BF00799228
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00799228