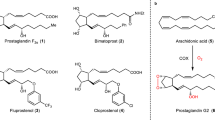
Abstract
Methods for the preparation of synthons for syntheses of spiro[2.4]heptane analogues of prostaglandins are described. Two of them (1a and1b) enable the syntheses of 11-deoxy-type compounds and were prepared from spiro[2.4]heptan-4-one (3) which after transformation into the 5-phenylthio-α,β-unsaturated ketone5 was subjected to conjugate addition of organocuprate reagent6. The third synthon (2)-a potential intermediate in syntheses of complete spiro[2.4]heptane analogues of prostaglandins-was prepared from the bicyclic ketone10 byBaeyer-Villiger oxidation followed by epoxidation.
Zusammenfassung
Es werden Synthesewege für Spiro[2.4]hepane als Analoge zu Prostaglandinen beschrieben. Zwei davon (1a und1b) ermöglichen die Synthese von Verbindungen des 11-Deoxy-Typs; sie wurden aus Spiro[2.4]heptan-4-on (3) dargestellt, das nach der Umwandlung zum 5-phenylthio-α,β-ungesättigten Keton5 einer konjugierten Addition von Organocuprat-Reagens6 unterworfen wurde. Das dritte (2), ein potentielles Zwischenprodukt in der Synthese von vollständigen Spiro[2.4]heptan-Analogen zu Prostaglandinen, wurde aus dem bicyclischen Keton10 durchBaeyer-Villiger-Oxidation gefolgt von einer Epoxidierung dargestellt.
Similar content being viewed by others
References
Hamon A.,Lacoume B.,Pasquet G.,Pilgvim W. R., Tetrahedron Lett.1976, 211.
Plantema O. G.,de Koning H.,Huisman H. O., J. C. S. Perkin I1978, 304.
Kurozumi S.,Toru T.,Tanaka T.,Kobayashi M.,Miura S.,Ishimoto S., Tetrahedron Lett.1976, 4091.
Toru T.,Kurozumi S.,Tanaka T.,Miura S.,Kobayashi M.,Ishimoto S., Tetrahedron Lett.1976, 4087.
Corey E. J.,Nicolaou K. C.,Beams D. J., Tetrahedron Lett.1974, 2439.
Crandall J. K., Seidewand R. J., J. Org. Chem.35, 697 (1970).
Monteiro H. J.,De Souza J. P., Tetrahedron Lett.1975, 921.
Monteiro H. J.,Gemal A. L., Synthesis1975, 437.
Coates R. M.,Pigott H. D.,Ollinger J., Tetrahedron Lett.1974, 3955.
Makosza M., Polish Patent1968, 55571 [Chem. Abstr.70, 106047 (1969)].
Brook P. R.,Harrison J. M., J.C.S. Perkin I1974, 778.
Kelly R. C., Van Rheenen V., Schletter I., Pillai M. D., J. Amer. Chem. Soc.95, 2746 (1973).
Corey E. J.,Arnold Z.,Hutton J., Tetrahedron Lett.1970, 307;Grieco P. A., J. Org. Chem.37, 2363 (1972).
Corey E. J.,Noyori R., Tetrahedron Lett.1970, 311.
Phillips B., Starcher P. S., Ash B. D., J. Org. Chem.23, 1823 (1958).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jarowicki, K., Jaworski, T. Synthons for syntheses of spiro[2.4]heptane analogues of prostaglandins. Monatsh Chem 115, 605–612 (1984). https://doi.org/10.1007/BF00799169
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00799169