Abstract
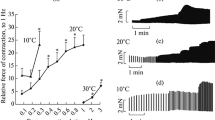
Spontaneous diastolic myofilament motion of the isolated ischemic-reperfused rat heart was studied by the technique of laser spectroscopy. Scattered light intensity fluctuations (SLIF) from the exposed surface of the left ventricle of the quiescent perfused (Langendorff) rat heart were quantified by the autocorrelation function (R1F: frequency weighted by power), and by determining the average power of the SLIF signal (PS). The stabilized mean control (±SE) R1F (mV2/s2) and PS (mV2/s) values were: 0.87±0.07 and 21.3±1.5 respectively. SLIF were characterized to index the extent of cell Ca2+-loading and the integrity of the sarcoplasmic reticulum (SR) functions by low Na+ and ryanodine perfusions. Low Na+ perfusion significantly increased the R1F values and produced pronounced spectral peaks between 0.5 and 2.5 Hz frequency bands; whereas ryanodine (1 μM) perfusion completely abolished the SLIF signals. Ischemia (34°C, 60 min.) produced nearly a 12-fold increase in the R1F and PS values accompanied by a four-fold increase in the left ventricular end-diastolic pressure (LVEDP) during the reperfusion period (34°C, 30 min.) Pronounced reperfusion SLIF peaks were evident at the frequency bands between 0.25–5.0 Hz. Hypothermic (10°C) preservation during ischemia reduced the frequency and the amplitude of the SLIF signals at various frequencies and prevented the rise in LVEDR. As compared to hypothermia alone, hypothermic cardioplegia offered a slightly better preservation. But hypothermia alone or in combination with cardioplegia failed to completely normalize the post-ischemic R1F and PS values. The results of this study indicate that hypothermic cardioplegia reduced the occurrence of spontaneous diastolic intracellular Ca2+ oscillations of the reperfused rat heart, but failed to completely normalize them.
Similar content being viewed by others
References
Abdulla AGH, Juggi JS, Bhatia KS (1988) Laser spectroscopy of quiescent perfused rat heart after ischemic arrest and cardioplegia. J Mol Cell Cardiol 20 (Suppl III): 2
Allen DG, Cairns SP, Turvey SE, Lee JA (1993) Intracellular calcium and myocardial function during ischemia. In: Sideman S, Beyar R. Interactive phenomena in the cardiac system. Plenum Press, New York pp 19–29
Bers DM (1987) Mechanisms contributing to the cardiac inotropic effect on Na pump inhibition and reduction of extracellular Na. J Gen Physiol 90: 497–504
Buckberg GD (1979) A proposed “solution” to cardioplegic controversy. J Thorac Cardiovasc Surg 77: 803–815
Capogrossi MC, Houser ST, Bahinski A, Lakatta EG (1987) Synchronous occurrence of spontaneous localized calcium release from the sarcoplasmic reticulum generates action potentials in rat cardiac ventricular myocytes at normal resting membrane potential. Cir Res 61: 498–503
Chiesi M, Ho MM, Inesi G, Somlyo AV, Somlyo AP (1981) Primary role of sarcoplasmic reticulum in phasic contractile activation of cardiac myocytes with shunted myolemma. J Cell Biol 91: 728–742
Fabiato A, Fabiato F (1972) Excitation-contraction coupling of isolated cardiac fibers with disrupted or closed sarcolemma. Calcium-dependent cyclic and tonic contractions. Cir Res 31: 293–307
Fabiato A, Fabiato F (1975) Contraction induced by a calcium triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol 469–495
Feher J, Briggs FN, Hess ML (1980) Characterization of cardiac sarcopiasmic reticulum from ischemic myocardium: Comparison of isolated sarcoplasmic reticulum with unfractioned homogenates. J Mol Cell Cardiol 12: 427–432
Ferrara N, Abete P, Longobardi G, Leoscod G, Caccese P, Leonarda De Rosa M, Orlando M, Fittipaldi R, Rengo F (1988) Action of Magnesium salts on the toxic effects of calcium overload in the isolated and perfused rat heart. G Ital Cardiol 18: 605–614
Flaherty JT, Schaff HV, Goldman RA, Gott VL (1979) Metabolic and functional effects of progressive degrees of hypothermia during global ischema. Am J Physiol 236: H839-H845
Fukumoto K, Takenaka H, Onitsuka T, Koga Y, Hamada M (1991) Effect of hypothermic ischemia and reperfusion on calcium transport by myocardial sarcolemma and sarcoplasmic reticulum. J Mol Cell Cardiol 23: 525–535
Gillette PC, Pinsky WW, Lewis RM, Bornek EP, Ward JM, Entman ML, Schwart A (1979) Myocardial depression after selective ischemic arrest. Subcellular Biochemistry 77 (4): 608–618
Golovina VA, Rozenshtraukh LV, Solov'ev BS, Undrovinas AI, Chernaya GG (1986) Wavelike spontaneous contractions of isolated myocytes. Biophysics 31: 311–318
Hansford RG, Lakatta EG (1987) Ryanodine release calcium from sarcoplasmic reticulum in calcium-tolerant rat cardiac myocytes. J Physiol 390: 453–467
Hess ML, Okabe E, Kontos HA (1981) Proton and free oxygen radical interaction with the calcium transport system of cardiac sarcoplasmic reticulum. J Mol Cell Cardiol 13: 767–772
Juggi JS (1985) Role of cardioplegia in cardiac stress during surgery. In: Stress and Heart Disease ed by Beamish RE, Singal PK, Dhalla NS. Martinus Nijhoff publishing, Boston, pp 364–385
Juggi JS, Yousof AM, Shuhaiber HJ, Abdulla AGH, Bhatia KS (1989) Cardioplegia and Cellular Calcium Homeostasis. In: Pathophysiology and Pharmacology of heart disease, ed by Anand IS, Wahi PL, Dhalla NS. Kluwer Academic Publishers, Boston, pp 81–90
Juggi JS, Bhatia KS, Abdulla AGH, Abutahun I, Ghaaedi F (1990) Oxygen free radicals increase intracellular calcium cycling detected by laser spectroscopy of quiescent perfused rat heart. J Mol Cardiol 22 (suppl I): 110A
Juggi JS, Ghaaedi FK, Bhatia KS, Owunwanne A (1993) Activated polymorphonuclear leucocytes (PMNs) enhance the Ca2+-dependent mechanical oscillations of the quiescent perfused rat heart. J Mol Cell Cardiol 25 (Suppl III): 187A
Kass RS, Tsien RW (1982) Fluctuations in membrane current driven by intracellular calcium in cardiac purkinje fibers. Biophys J 38: 259–269
Kort AA, Capogrossi MC, Lakatta EG (1985) Frequency, Amplitude, and Propagation Velocity of Spontaneous Ca++-Dependent Contractile Waves in Intact Adult Rat Cardiac Muscle and Isolated Myocytes. Circ Res 57: 844–855
Lakatta EG, Lappe DL (1981) Diastolic scattered light fluctuations, resting force and twitch force in mammalian cardiac muscle. J Physiol 315: 369–394
Lakatta EG, Capogrossi MC, Kort AA, Stern MD (1985) Spontaneous myocardial Ca2+-oscillations: An overview with emphasis on ryanodine and caffeine. Federation Proc 44: 2977–2983
Lappe DL, Lakatta EG (1980) Intensity Fluctuation Spectroscopy Monitors Contractile Activation in “Resting” Cardiac Muscle. Science 207: 1369–1371
Lee JA, Allen DG (1992) Changes in intracellular free calcium concentration during long exposures to simulated ischemia in isolated mammalian ventricular muscle. Circ Res 71: 59–69
Lee JA, Allen DG (1991) Mechanisms of acute ischemic contractile failure of the heart: role of intracellular calcium. J Clin Invest 88: 361–367
Mulder BJM, De Tombe PP, ter Keurs EEJ (1989) Spontaneous and Propagated Contractions in Rat Cardiac Trabeculae. J Gen Physiol 93: 943–961
Stern MD, Kort AA, Bhatnagar GM, Lakatta EG (1983) Scattered-light Intensity Fluctuations in Diastolic Rat Cardiac Muscle caused by Spontaneous Ca2+-dependent Cellular Mechanical Oscillations. J Gen Physiol 82: 119–153
Stern MD, Weisman HF, Renlund DG, Gerstenblith G, Hano O, Blank PS, Lakatta EG (1989) Laser backscatter studies of intracellular Ca2+-oscillations in isolated hearts. Am J Physiol 257: H665–673
Takamatsu T, Weir WG (1990) Calcium waves in mamalian heart: quantiticadom of origin, magnitude, waveform, and velocity. FASEB J 4: 1519–1525
Weiss RG, Gerstenblith G, Lakatta EG (1990) Calcium oscillations index the extent of calcium loading and predict functional recovery during reperfusion in rat myocardium. J Clin Invest 85: 757–765
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Juggi, J.S., Abdulla, A.G.H., Bhatia, K.S. et al. Hypothermic cardioplegia reduces the occurrence of spontaneous diastolic myofilament motion of the ischemic-reperfused rat heart. Basic Res Cardiol 90, 314–322 (1995). https://doi.org/10.1007/BF00797909
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00797909