Conclusions
-
1.
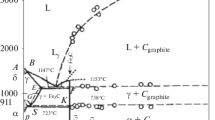
A study was made of the dissolution mechanism of Cr3C2 in iron. It was established that the reaction of orthorhombic chromium carbide with iron begins at 350–400°C and, up to the beginning ofα→γ transformation, manifests itself in diffusional exchange of iron and chromium atoms and in the formation of a special orthorhombic carbide. In the temperature range of existence ofγ-iron, the dissolution of the orthorhombic carbide is preceded by its recrystallization into the hexagonal form and the latter's enrichment in iron to a content close to the solubility limit of iron.
-
2.
Up to a temperature of ∼1050°C, the carbide dissolution process is limited by the (CrFe)3C2→ (CrFe)7C3 transformation. Above 1050°C, diffusion becomes the link controlling the rate of the process.
-
3.
Orthorhombic chromium carbide dissolves quite rapidly in iron, and can therefore be employed as an alloying addition.
Similar content being viewed by others
Literature cited
N. F. Vyaznikov, Alloy Steel [in Russian], Metallurgizdat (1963).
I. D. Radomysel'skii and V. A. Dymchenko, Poroshkovaya Met., No. 9 (1969).
Author information
Authors and Affiliations
Additional information
Translated from Poroshkovaya Metallurgiya, No. 3 (99), pp. 88–92, March, 1971.
Rights and permissions
About this article
Cite this article
Radomysel'skii, I.D., Dymchenko, V.A. Mechanism and kinetics of dissolution of Cr3C2 in iron. Powder Metall Met Ceram 10, 240–243 (1971). https://doi.org/10.1007/BF00796718
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00796718