Abstract
Background
The mitogenactivated protein kinase kinase (MAPKK) is a protein downstream ras which is rapidly activated in cells stimulated with various extracellular signals. These proteins are believed to play a pivotal role in integrating and transmitting transmembrane signals required for cell growth.
Methods and Results
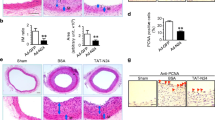
To study the effect of inhibition of MAPKK on smooth muscle cell (SMC) proliferationin vivo after vascular injury, we performed experimental balloon angioplasty using the standard Clowes technique in male Wistar rats 14-weeks old. The animals did not receive any treatment after vascular injury (N=6) or were randomly assigned to receive, after balloon injury, a 30% (w/v) pluronic gel solution applied to the injured carotid artery, containing respectively: 1) no plasmid DNA (n=10); 2) RSV-lacZ (encoding the β-galactosidase gene) as control gene without effects on SMC proliferation (n=10); 3) Tg-CAT (encoding cloramphenicol acetyl-transferase gene under the control of thyreoglobulin promoter) as an additional control gene without effects on SMC proliferation (n=7); 4) a negative mutant of Mitogen-Activated Protein Kinase Kinase (MAPKK) (n=13). Fourteen days after vascular injury, carotid arteries were removed and cross sections were cut and stained with hematoxylin/eosin. Morphometric analysis demonstrated, in the MAPKK-treated rats, a significant reduction of both neointima (0.096±0.018 mm2 vs. 0.184±0.019 mm2, p<0.01) and neointima/media ratio (0.603±0.103 vs. 1.147±0.161, p<0.01) compared to control DNA.
Conclusions
The inhibition of MAPKK, by a dominant inhibitor mutant gene, prevents the SMC proliferation after vascular injuryin vivo.
Similar content being viewed by others
References
Alessi DR, Saito Y, Campbell DG, Cohen P, Sithanandam G, Rapp U, Ashworth A, Marshall CJ, Cowley S (1994) Identification of the sites in map kinase kinase-1 phosphorylated by p74raf-1. EMBO J 13; 7: 1610–1619
Blenis J (1993) Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci USA 90 (13): 5889–5892
Blumer KJ, Johnson GL (1994) Diversity in function and regulation of MAP kinase pathway. Trends Biol Sci 19; 236–240
Boguski MS, McCormick F (1993) Protein regulating ras and its relatives. Nature 366: 643–654
Bourne HR, Sanders DA, McCormick F (1991) The GTPase superfamily: Conserved structure and molecular mechanism. Nature 349: 117–127
Brott BC, Labinaz M, Culp SC, Fortin DF, Virmani R, Phillips HR, Stack RS (1994) Vessel remodeling after angioplasty: comparative anatomic studies. J Am Coll Cardiol 23: 138A. Abstract
Califf RM, Fortin DF, Frid DJ, Harlan WR III Ohman EM, Bengtson JR, Nelson CL, Tcheng JE, Mark DB, Stack RS (1991) Restenosis after coronary angioplasty: an overview. J Am Coll 17: 2B-13B
Clowes AW, Reidy MA, Clowes MM (1983) Kinetics of cellular proliferation after arterial injury: I. Smooth muscle growth in the absence of endothelium. Lab Invest 49: 327–333
Clowes AW, Reidy MA, Clowes MM (1983) Mechanisms of stenosis after arterial injury. Lab Invest 49: 208–215
Cowley S, Paterson H, Kemp P, Marshall CJ (1994) Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell 77: 841–852
Detre K, Holubkov R, Kelsey S, Cowley M, Kent K, Williams D, Myler R, Faxon D, Holmes D, Bourassa M, Block P, Gosselin A, Bentivolgio L, Leatherman L, Dorros G, King SB III, Galichia J, Al-Bassam M, Leon M, Robertson T, Passamani E (1988) Percutaneous transluminal coronary angioplasty in 1985–1986 and 1977–1981: the National Heart Lung and Blod Institute Registry. N Engl J Med 318: 265–270
Dickson B, Sprenger F, Morrison D, Hafen E (1992) Raf functions downstream of Ras1 in the Sevenless signal transduction pathway. Nature 360: 600–603
DiMario C, Gil R, Camezind E, Ozaki Y, von Birgelen C, Umans V, de Jaegere P, de Feyter PJ, Roelandt JRTC, Serruys PW (1995) Quantitative assessment with intracoronary ultrasound of the mechanism of restenosis after percutaneous transluminal coronary angioplasty and directional coronary atherectomy. Am J Cardiol 75: 772–777
Dixon WJ, Massey J Jr (1969)Introduction to Statistical Analysis. New York, Mc Graw-Hill, pp 231–234
Fischmann DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, Detre K, Veltri L, Ricci D, Nobuyoshi M, Cleman M, Heuser R, Almond D, Teirstein PS, Fisch RD, Colombo A, Brinker J, Moses J, Shanknovich A, Hirshfeld J, Bailey S, Ellis S, Rake R, Goldberg S (1994) for the Stent Retsenosis Study Investigators. N Engl J Med 331: 496–501
Furchgott RF (1983) Role of endothelium in responses of vascular smooth muscle. Circ Res 53: 557–573
Galis Z, Sukhova G, Larck M, Libby P (1994) Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 94: 2493–2503
Gerald M, Tschirky H (1969) Measurement of blood pressure in unanesthetized rats and mice. Drug Res 18: 1285–1287
Gibbons G, Dzau V (1994) The emerging concept of vascular remodeling. N Engl J Med 330: 1431–1438
Glagov S (1994) Intimal hyperplasia, vascular remodeling, and the restenosis problem. Circulation 89: 2888–2891
Gruentzig AR, King SB, Sclumpf M, Siegenthaler W (1987) Long-term follow-up after percutaneous transluminal coronary angioplasty. N Engl J Med 316: 1127–1132
Halberg B, Rayter SI, Downard J (1994) Interaction of ras and raf in intact mammalian cells upon extracellular stimulation. J Biol Chem 269: 3913–3916
Hoffmann R, Mintz GS, Dussailant GR, Popma JJ, Pichard AD, Satler LF, Kent KM, Griffin J, Leon MB (1996) Patterns and mechanisms of stent restenosis. Circulation 94: 1247–1254
Holmes DR Jr, Vlietstra RE, Smith HC, Vetrovec GW, Kent KM, Cowley MJ, Taxon DP, Gruentzig AR, Kelsey SF, Detre KM, van Raden MJ, Mock MB (1984) Restenosis after percutaneous transluminal coronary angioplasty (PTCA): a report from the PTCA Registry of the National Heart, Lung, and Blood Institute. Am J Cardiol 53: Suppl: 77C-81C
Indolfi C, Avvedimento EV, Di Lorenzo E, Esposito G, Rapacciuolo A, Giuliano P, Grieco D, Cavuto L, Stingone AM, Ciullo I, Condorelli GL, Chiariello M (1997) Activation of cAMP-PKA signalling in vivo inhibits smooth muscle cell proliferation induced by vascular injury. Nature Medicine 3: 775–779
Indolfi C, Avvedimento EV, Rapacciuolo A, Di Lorenzo E, Esposito G, Stabile E, Feliciello A, Mele E, Giuliano P, Condorelli GL, Chiariello M (1995) Inhibition of cellular RAS prevents smooth muscle cell proliferation after vascular injury in vivo. Nature Medicine 6: 541–545
Indolfi C, Chiariello M, Avvedimento EV (1996) Selective gene therapy for proliferative disorders: sence and antisense. Nature Med 2: 634–635
Indolfi C, Esposito G, Di Lorenzo E, Rapacciuolo A, Feliciello A, Porcellini A, Avvedimento EV, Condorelli M, Chiariello M (1995) Smooth muscle cell proliferation is proportional to the degree of balloon injury in a rat model of angioplasty. Circulation 92: 1230–1235
Kolch W et al. (1993) Protein kinase C activates Raf-1 by direct phosphorilation. Nature 364: 249–252
Lafont A, Guzman L, Whitlow P, Goormastic M, Cornhill J, Chisholm G (1995) Restenosis after experimental angioplasty: intimal, medial, and advential changes associated with constrictive remodeling. Circ Res 76: 996–1002
Langille BL, Bendeck MP, Keeley FW (1989) Adaptations of carotid arteries of young and mature rabbits to reduced carotid flow. Am J Physiol 256: H931-H939
Langille BL, O'Donnel F (1986) Reductions in arterial diameter produced by cronic decreases in blood flow are endothelium dependent. Science 231: 405–407
Liu MW, Roubin GS, King SB III (1989) Restenosis after coronary angoplasty: potential biologic determinants and role of intimal hiperplasia. Circulation 79: 1374–1387
Marshall CJ (1995) Specifity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signalregulated kinase activation. Cell 80: 179–185
Mintz GS, Pompa JJ, Pichard AD, Kent KM, Satler LF, Wong SC, Hong MK, Kovach JA, Leon MB (1996) Arterial remodeling after coronary angioplasty. A serial intravascular ultrasound study. Circulation 94: 35–43
Nobuyoshi M, Kimura T, Nosaka H, Mioka S, Ueno K, Yokoi H, Hamasaki N, Horiuchi H, Ohishi H (1988) Restenosis restenosis after successful percutaneous transluminal coronary angioplasty: serial angiographic follow-up of 229 patients. J Am Coll 12: 616–623
Nunes GL, Thomas CN, Hanson SR, Barry JJ, King SB 3rd Scott NA (1995) Inhibition of platelet-dependent thrombosis by local delivery of heparin with a hydrogel-coated balloon. Circulation 92: 1697–1700
Porras A, Muszyneski K, Rapp UR, Santos E (1994) Dissociation between activation of Raf-1 kinase and the 42-kDa mitogen-activated protein kinase/90-kDa S6 kinase (MAPK/RSK) cascade in the insulin/ras pathway of adypocytic differentiation of 3T3 L1 cells. J Biol Chem 269 (17): 12741–12748
Post MJ, Borst C, Kuntz RE (1994) The relative importance of arterial remodeling compared with intimal hyperplasia in lumen renar-rowing after ballon angioplasty. Circulation 89: 2816–2821
Reidy MA, Fingerle J, Linder V (1992) Factors controlling the development of arterial lesions after injury. Circulation 86 (suppl III): III-43–III-46
Roberts TM (1992) Cell biology. A signal chain of events. Nature 360: 534–535
Schwartz RS, Holmes DR jr, Topol EJ (1992) The restenosis paradigm revisited: an alternative proposal for cellular mechanism. J Am Coll Cardiol 20: 1284–1293
Serruys PW, De Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, Emanuelsson H, Marco J, Legrand V, Materne P, Belardi J, Sigwart U, Colombo A, Goy JJ, van den Heuvel P Delcan J, Morel MA for the Benestent Study Group (1994) A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med 331: 489–495
Serruys PW, Emanuelsson H, van der Giessen W, Lunn AC, Kiemeney F, Macaya C, Rutsch W, Heyndrickx G, Suryapranata H, Legrand V, Goy JJ, Materne P, Bonnier H, Morice MC, Fajadet J, Belardi J, Colombo A, Garcia E, Ruygrok P, de Jaegere P, Morel MA on behalf of the Benestent II Study Group (1996) Heparin-coated Palmaz-Schatz stents in human coronary arteries. Early outcome of the Benestent II pilot study. Circulation 93: 412–422
Serruys PW, Luijten HE, Beatt KJ, Jeuskens R, De Feyter PJ, Van den Brand M, Reiber JHC, ten Katen HJ, van Es GA, Hugenoltz PG (1988) Incidence of restenosis after successful coronary angioplasty: a time-related phenomenon: a quantitative angiographic study in 342 consecutive patients at 1,2,3, and 4 months. Circulation 77: 361–371
Simons M, Edelman ER, DeKeyser JL, Langer R, Rosenberg RD (1992) Antisense c-myb oligonucleotides inhibits intimal arterial smooth muscleell accumulation in vivo. Nature 359: 67–70
Strauss BH, Chilsolm RJ, Keeley FW, Gotlieb AI, Logan RA, Armstrong PW (1994) Extracellular matrix remodeling after ballon angioplasty injury in a rabbit model of restenosis. Circ Res 75: 650–658
Williams NG, Paradis H, Agarwal S, Charest DL, Pelech SL, Roberts TM (1993) Raf-1 and p21v-ras cooperate in the activation of mitogen-activated protein kinase. Proc Natl Acad Sci USA 90: 5772–5776
Wood KW, Sarnecki C, Roberts TM, Blenis J (1992) Ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell 68 (6): 1041–1050
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Indolfi, C., Avvedimento, E.V., Rapacciuolo, A. et al. In vivo gene transfer: prevention of neointima formation by inhibition of mitogen-activated protein kinase kinase. Basic Res Cardiol 92, 378–384 (1997). https://doi.org/10.1007/BF00796211
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00796211