Conclusions
An investigation was carried out into the kinetics of the process of formation of manganese nitrides in nitrogen and ammonia streams. It was established that the rate of formation of manganese nitrides is higher in ammonia than in nitrogen. The kinetic parameters of manganese nitride formation were calculated.
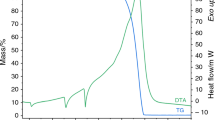
It is shown that in nitrogen the only manganese nitride that can be synthesized in the pure form is Mn4N. In ammonia, depending on the temperature and duration of the nitriding process, it is possible to obtain MnN, Mn2N, and Mn3N2 in the pure form, free from any other phases.
A study was made of the chemical stability of manganese nitrides of compositions Mn4N and Mn3N2 in water and in concentrated and dilute mineral acids. It was established that these two nitrides are stable in water. In mineral acids they dissolve, the rate of dissolution being higher in concentrated than in dilute acids. In nitric acid Mn4N and Mn3N2 decompose with the liberation of part of the nitrogen in molecular form.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Translated from Poroshkovaya Metallurgiya, No. 3(171), pp. 65–70, March, 1977.
Rights and permissions
About this article
Cite this article
Lyutaya, M.D., Goncharuk, A.B. Manganese nitrides. Powder Metall Met Ceram 16, 208–212 (1977). https://doi.org/10.1007/BF00794089
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00794089