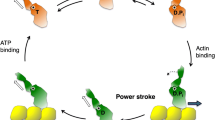
Abstract
In this chapter we consider a current perception of the molecular mechanisms controlling myofilament activation with emphasis on alterations that may occur in familial hypertrophic cardiomyopathy (FHC). FHC is a sarcomeric disease (100) with an autosomal dominant pattern of heritability (27, 51). There is a substantial body of evidence implicating missense mutations in the β-MHC gene as causal for the development of this disease. Recently, mutations in genes of two thin filament regulatory proteins, cardiac troponin T (cTnT) and α-tropomyosin (α-Tm), have also been linked to FHC. The commonality among the functional consequences of these mutations remains an important question. This review discusses how these pathological mutations may impact the activation process by disrupting critical structure function relations in both the thick and thin filaments.
Similar content being viewed by others
References
Al-Mahdawi S, Chamberlain S, Cleland J, Nihoyannopoulos P, Gilligan D, French J, Choudhurv L, Williamson R, Oakley C (1993) Identification of a mutation in the β-cardiac myosin heavy chain gene in a family with hypertrophic cardiomyopathy. Br Heart J 69: 136–141
Anan R, Greve G, Thiefelder L, Watkins H, McKenna WJ, Solomon S, Vecchio C, Shonom H, Nakao S, Tanaka H, Mares A Jr, Towbin JA, Spirito P, Roberts R, Seidman JG, Seidman CE (1994) Prognostic implications of novel β-cardiac myosin heavy chain gene mutations that cause familial hypertrophic cardiomyopathy. J Clin Invest 93: 280–285
Breitbart RE, Nadal-Ginard B (1986) Complete nucleotide sequence of the fast skeletal troponin T gene. J Mol Biol 188: 313–324
Breitbart RE, Nguyen HT, Medford RM, Destree AT, Mahdavi V, Nadal-Ginard B (1985) Intricate combinatorial patterns of exon splicing generate multiple regulated troponin T isoforms from a single gene. Cell 41: 67–82
Brisson JR, Golosinska K, Smillie LB, Sykes BD (1986) Interaction of tropomyosin and troponin T: a proton nuclear magnetic resonance study. Biochemistry 25: 4548–4555
Burke AP, Farb A, Virmani R, Goodman J, Smialek JE (1991) Sports-related and non-sports-relared sudden cardiac death in young adults. Am Heart J 121: 568–575
Carrier L, Hengstenberg C, Beckmann JS, Guicheney P, Dufour C, Bercovici J, Dausse E, Berebbi-Betrand I, Wisnewsky C, Pulvenis D, Fetler L, Vignal A, Weissenbach J, Hillaire D, Feingold J, Bouhour JB, Hagege A, Desnos M, Isnard R, Dubourg O, Komajda M, Schwartz K (1993) Mapping of a novel gene for familial hypertrophic cardiomyopathy to chromosome 11. Nature Genetics 4: 311–313
Caspar DLD, Cohen C, Longley W (1969) Tropomyosin: crystal structure, polymorphism and molecular interactions. J Mol Biol 41: 87–107
Cho YJ, Liu J, Hitchcock-DeGregori SE (1990) The amino terminus of muscle tropomyosin is a major determinant for function. J Biol Chem 265: 538–545
Chong PCS, Hodges RS (1982) Photochemical cross-linking between rabbit skeletal troponin subunits. J Biol Chem 257: 11667–11672
Chong PCS, Hodges RS (1982) Photochemical cross-linking between rabbit skeletal troponin and α-tropomyosin. J Biol Chem 257: 9152–9160
Cohen C, Szent-Gyorgyi AG (1957) Optical rotation and helical polypeptide chain configuration in α-proteins. J Amer Chem Soc 79: 248
Cohen C, Caspar DLD, Parry DAD, Lucas RM (1971) Tropomyosin crystal dynamics. Cold Spring Harbor Symp Quant Biol 36: 205–216
Consevage MW, Salada GC, Baylen BG, Ladda RL, Rogan PK (1994) A new missense mutation, Arg 719 Gln, in the β-cardiac heavy chain myosin gene of patients with familial hypertrophic cardiomyopathy. Human Mol Genetics 3: 1025–1026
Cotran RS, Kumar V, Robbins SL (eds) (1989) The Heart. In. Pathologic Basis of Disease 4th edition. WB Saunders Company, Philadelphia, pp 642–656
Cuda G, Fanananpazir L, Zhu W, Sellers JR, Epstein ND (1993) Skeletal muscle expression and abnormal function of β-myosin in hypertrophic cardiomyopathy. J Clin Invest 91: 2861–2865
Durand JB, Anchee AB, Roberts R (1995) Molecular and clinical aspects of inherited cardiomyopathies. Annals of Medicine 27: 311–317
Epstein ND, Cohn GM, Cyrate L, Fananapazir L (1992) Differences in clinical expression of hypertrophic car diomyopathy associated with two distinct mutations in the β-myosin heavy chain gene a 908Leu-Val mutation and a 403Arg-Gln mutation. Circulation 86: 345–352
Epstein ND, Fananapazir L, Lin HJ, Mulvihill J, White R, Lalouel JM, Lifton RP, Nienhuis AW, Leppert M (1992) Evidence of genetic heterogeneity in five kindreds with familial hypertrophic cardiomyopathy. Circulation 85: 635–647
Fananapazir L, Epstein ND (1995) Prevalence of hypertrophic cardiomyopathy and limitations of screening methods. Circulation 92: 700–704
Fananapazir L, Dalakas MC, Cyran F, Cohn G, Epstein ND (1993) Missense mutations in the β-myosin heavy-chain gene cause central core dise hypertrophic cardiomyopathy. Proc Natl Acad Sci USA 90: 3993–3997
Fananapazir L, Epstein ND (1994) Genotype-phenotype correlations in hypertrophic cardiomyopathy. Circulation 89: 22–32
Farah CS, Reinach FC (1995) The troponin complex and regulation of muscle contraction. Faseb J 9: 755–767
Flicker PF, Phillips GN JR, Cohen C (1982) Troponin and its interactions with tropomyosin. J Mol Biol 162: 495–501
Fryberg E, Fryberg CC, Beall C, Saville DL (1990) Drosophila melanogaster troponin-T mutations engender three distincct syndromes of myofibrillar abnormalities. J Mol Bio 216: 657–675
Geisterfer-Lowrance AAT, Kass S, Tanigawa G, Vosberg H, McKenna W, Seidman CE, Seidman JG (1990) A molecular basis for familial hypertrophic cardiomyopathy: a β-myosin heavy chain gene missence mutation. Cell 62: 999–1006
Greaves SC, Roche AHG, Meutze JM, Whitlock RML, Veale AMO (1987) Inheritance of hypertrophic cardiomyopathy: a cross sectional and M mode echocardiographic study of 50 families. Br Heart J 58: 259–66
Gusev NB, Barskaya NV, Verin AD, Duzhenkova IV, Khuchua ZA, Zheltova AO (1983) Some properties of cardiac troponin T structure. Biophys J 213: 123–129
Heeley DH, Smillie LB (1988) Interaction of rabbit skeletal muscle troponin T and F-actin at physiological ionic strength. Biochemistry 27: 8227–8232
Heeley DH, Golosinska K, Smillie LB (1987) The effects of troponin T fragments T1 and T2 on the binding of nonpolymerizable tropomyosin to F-actin in the presence and absence of troponin I and troponin C. J Biol Chem 262: 9971–9978
Heeley DH, Smillie LB, Lohmeirer-Vogel EM (1989) Effects of deletion of tropomyosin overlap on regulated actomyosin overlap on regulated actomyosin subfragment 1 ATPase. Biochem J 258: 831–836
Hejtmancik JF, Brink PA, Towbin J, Hill R, Brink L, Tapscott T, Trakhtenbroit A, Robert R (1991) Localization of gene for familial hypertrophic cardiomyopathy to chromosome 14q1 in a diverse US population. Circulation 83: 1592–1597
Hitchcock SE (1975) Regulation of muscle contraction: binding of troponin and its components to actin and tropomyosin. Eur J Biochem 52: 255–263
Hitchcock SE, Zimmerman CJ (1981) Study of the structure of troponin T by measuring the relative reactivities of lysines with acetic anhydride 147: 125–151
Hodges RS, Sodek J, Smillie LB, Jurasek L (1972) Tropomyosin: amino acid sequence and coiled-coil structure Cold Spring Harbor Symp Quant Biol 37: 299–310
Iio T (1985) Conformational changes of troponin T induced by calcium binding to troponin C. J Biochem 98: 261–263
Ishii Y, Lehrer SS (1991) Two-site attachment of troponin to pyrenelabelled tropomyosin. J Biol Chem 266: 6894–6903
Jarcho JA, McKenna W, Pare P, Solomon SD, Holcombe RF, Dickie S, Levi T, Donis-Keller H, Seidman JG, Seidman CE (1989) Mapping a gene for familial hypertrophic cardiomyopathy to chromosome 14q1. N Engl J Med 321: 1372–1378
Jin JP, Lin JJC (1989) Isolation and characterization of cDNA clones encoding embryonic and adult isoforms of rat cardiac troponin T. J Biol Chem 264: 14471–14477
Leger J, Bouveret P, Schwartz K, Swynghedauw B (1976) A comparative study of skeletal and cardiac tropomyosins. Pflugers Archiv 362: 271–277
Lehman W, Craig R, Vilbert P (1994) Ca2+-induced tropomyosin movement in Limulus thin filaments revealed by three dimensional reconstruction. Nature 368: 65–67
Lehrer SS (1994) The regulatory switch of the muscle thin filament: Ca2+ or myosin heads? J Muscle Res and Cell Mot 15: 232–236
Lowey S, Slayter HS, Weeds AG, Baker H (1969) Substructure of the myosin molecule. J Mol Biol 42: 1–29
Mak AS, Smillie LB (1981) Nonpolymerizable tropomyosin: preparation some properties and F-actin binding. 101: 208–214
Mak AS, Smillie LB (1981) Structural interpretation of the two site binding of troponin on the muscle thin filament. J Mol Bio 149: 541–550
Mak AS, Smillie LB, Stewart GR (1980) A comparison of the amino acid of rabbit skeletal muscle α- and β-tropomyosins. J Biol Chem 255: 3647–3651
Marian AJ, Yu QT, Mann DL, Graham FL, Roberts R (1995) Expression of a mutation causing hypertrophic cardiomyopathy disrupts sarcomere assembly in adult feline myocytes. 77: 98–106
Marian AJ, Yu QT, Mares A, Hill R, Roberts R, Perryman MB (1992) Detection of a new mutation in the β-myosin heavy chain gene in an individual with hypertrophic cardiomyopathy. J Clin Invest 90: 2156–2165
Marion AJ, Robert R (1995) Recent advances in molecular genetics of hypertrophic cardiomyopathy. Circulation 92: 1336–1347
Maron BJ, Epstein SE, Roberts WC (1985) Causes of sudden death in competitive athletes. J Am Coll Cardiol 7: 204–214
Maron BJ, Nichols PF III, Pickle LW, Wesley YE, Mulvihill JJ (1984) Patterns of inheritance in hypertrophic cardiomyopathy assessment by M mode and two dimensional echocal diography. Am J Cardiol 53: 1087–1094
McKenna WJ, Camm AJ (1989) Sudden death in hypertrophic cardiomyopathy. Circulation 80: 1489–1492
McLachalan AD, Stewart M, Smillie LB (1975) Sequence repeats in α-tropomyosin. J Mol Biol 98: 281–291
McLachalan AD, Stewart M (1975) Tropomyosin coiled-coil interactions: evidence for an unstaggered structure. J Mol Biol 98: 293–304
Medford RM, Nguyen HT, Destree AT, Summers E, Nadal-Ginard B (1984) A novel mechanism of alternative RNA splicing for the developmentally regulated generation of troponin T isoforms from a single gene. Cell 38: 409–421
Millar NC, Homsher E (1990) The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. J Biol Chem 265: 20234–20240
Morris EP, Lehrer SS (1984) Troponin-tropomyosin interactions. Flourescence studies of the binding of troponin, troponin T, and chymotryptic troponin T fragments to specifically labeled tropomyosin. Biochemistry 23: 2214–2220
Moss RL (1992) Ca2+ regulation of mechanical properties of striated muscle. Cir Res 70: 865–884
Muthuchamy M, Grupp I, Grupp G, O'Toole BA, Kier AB, Boivin GP, Neumann J, Wieczorek DF (1995) Molecular and physiological effects of overexpressing striated muscle β-tropomyosin in the adult murine heart J Biol Chem: in Press
Nakajima-Taniguchi C, Matsue H, Nagata S, Kishmoto T, Yamauchi-Takihara K (1995) Novel missence mutation in α-tropomyosin gene found in japanese patients with hypertrophic cardiomyopathy. J Mol Cell Cardiol 27: 2053–2058
Nishi H, Kimura A, Harada H, Toshima H, Sasazuki T (1992) Novel missence mutation in cardiac β-myosin heavy chain gene found in a japanese patient with hypertrophic cardiomyopathy. Biochem Biophys Res Comm 188: 379–387
Ohtsuki I (1979) Molecular arrangement of troponin T in the thin filament. J Biochem 86: 491–497
Palmiter KA, Kitada Y, Muthuchamy M, Wieczorek DF, Solaro RJ (1996) Exchange of β- for α-tropomyosin in hearts of transgenic mice induces changes in thin filament response to Ca2+, strong cross-bridge binding, and protein phosphorylation. J Biol Chem 271: 11611–11614
Pan B, Potter JD (1992) Two genetically expressed troponin T fragments representing α and β isoforms exhibit functional differences. J Biol Chem 267: 23052–23056
Pan B, Gordon AM, Potter JD (1991) Deletion of the first 45 NH2-terminal residures of rabbit skeletal troponin T strengthens binding of troponin to immobilized tropomyosin. J Biol Chem 266: 12432–12438
Pan BS, Gordon AM, Luo Z (1989) Removal of tropomyosin overlap modifies cooperative binding of myosin S1 to reconstituted thin filaments of rabbit striated muscle. J Biol Chem 264: 8495–8498
Parry DAD (1981) Analysis of the amino acid sequence of α-tropomyosin-binding fragment from troponin T. J Mol Biol 146: 259–263
Parry DAD (1975) Analysis of the primary sequence of α-tropomyosin from rabbit skeletal muscle. J Mol Biol 98: 519–535
Parry DAD (1976) Movement of tropomyosin during regulation of vertebrate skeletal muscle: a simple physical model. Biochem Biophys Res Com 68: 323–328
Pato MD, Mak AS, Smillie LB (1981) Fragments of rabbit striated muscle α-tropomyosin. J Biol Chem 256: 602–607
Pato MD, Mak AS, Smillie LB (1981) Fragments of rabbit striated muscle α-tropomyosin. J Biol Chem 256: 593–598
Pearlstone JR, Smillie LB (1982) Binding of troponin-T fragments to several types of tropomyosin. J Biol Chem 257: 10587–10592
Pearlstone JR, Smillie LB (1983) Effects of troponin-I plus-C on the binding of troponin-T and its fragments to α-tropomyosin. J Biol Chem 258: 2534–2542
Pearlstone JR, Smillie LB (1981) Identification of a second binding region on rabbit skeletal troponin-T for α-tropomyosin. FEBS Letters 128: 119–122
Pearlstone JR, Smillie LB (1977) The binding site of rabbit skeletal α-tropomyosin on troponin T. Can J Biochem 55: 1032–1038
Pearlstone JR, Smillie LB (1985) The binding sites of rabbit skeletal troponin-I on troponin-T. Can J Biochem 58: 649–654
Pearlstone JR, Smillie LB (1985) The interaction of rabbit skeletal muscle troponin T fragments with troponin I. Can J Biochem Cell Biol 63: 212–218
Pearlstone JR, Smillie LB (1978) Troponin T fragments: physical properties and binding to troponin C. Can J Biochem Cell Biol 56: 521–527
Pearlstone JR, Carpenter MR, Smillie LB (1977) Primary structure of rabbit skeletal muscle troponin-T. J Biol Chem 252: 971–977
Pearlstone JR, Carpenter MR, Johnson P, Smillie LB (1976) Aminoacid sequence of tropomyosin-binding component of rabbit skeletal muscle troponin. Proc Nat Acad Sci USA 73: 1902–1906
Phillips GN Jr, Fillers JP, Cohen C (1986) Tropomyosin crystal structure and muscle regulation. J Mol Biol 192: 111–131
Phillips GN Jr, Fillers JP, Cohen C (1980) Motions of tropomyosin. Biophys J 32: 485–502
Potekhin SA, Privalov PL (1982) Cooperative blocks in tropomyosin. J Mol Biol 159: 519–535
Potter JD, Gergely J (1974) Troponin, tropomyosin, and actin interaction in the Ca2+ regulation of muscle contraction. Biochemistry 13: 2697–2703
Potter JD, Sheng Z, Pan B, Zhao J (1995) A direct regulatory role for troponin T and a dual role for troponin C in the Ca2+ regulation of muscle contraction. J Biol Chem 270: 2557–2562
Privalov PL (1982) Double-stranded coiled coils: tropomyosin, paramyosin, and the myosin rod. Advan Protein Chem 35: 31–55
Rayment I, Holden HM, Whitaker M, Yohn CB, Lorenz M, Holmes KC, Milligan RA (1993) Structure of the actin-myosin complex and its implications for muscle contraction. Science 261: 58–65
Risnik VV, Verin AD, Gusev NB (1985) Comparison of the structure of two cardiac troponin T isoforms. Biochem J 225: 549–552
Sodek J, Hodges RS, Smillie LB, Jurasek L (1972) Amino-acid sequence of rabbit skeletal tropomyosin and its coiled-coil structure. Proc Nat Acad Sci USA 69: 3800–3804
Solaro RJ, VanEyk JI (1996) Altered interactions among thin filament protein modulate cardiac function. J Mol Cell Cardio 28: 217–230
Solomon SD, Jarcho JA, McKenna W, Geisterfer-Lowrance A, Germain R, Salerni R, Seidman JG, Seidman CE (1990) Familial hypertrophic cardiomyopathy is a genetically heterogeneous disease. J Clin Invest 86: 998–999
Straceski AJ, Geisterfer-Lowrance A, Seidman CE, Seidman JG, Leinwand LA (1994) Functional analysis of myosin missense mutations in familial hypertrophic cardiomyopathy. Proc Nat Acad Sci 91: 589–593
Sweeney HL, Straceski AJ, Leinwand LA, Tikunov BA, Faust L (1994) Heterologous expression of a cardiomyopathic myosin that is defective in its actin interaction. J Biol Chem 269: 1603–1605
Swynghedauw B (1986) Developmental and functional adaptation or contractile proteins in cardiac and skeletal muscles. Physiol Reviews 66: 710–771
Tanigawa G, Jarcho JA, Kass S, Solomon SD, Vosberg H, Seidman JG, Seidman CE (1990) A molecular basis for familial hypertrophic cardio-myopathy: an α/β-cardiac myosin heavy chain hybrid gene. Cell 62: 991–998
Tanokura M, Ohtsuki I (1982) Location of troponin I-binding on troponin T sequence. FEBS Letters 145: 147–149
Tanokura M, Tawada Y, Onoyama Y, Nakamura S, Ohtsuki I (1981) Primary structure of chymotryptic subfragments from rabbit skeletal troponin T. J Biochem 90: 263–265
Tao T, Gong BJ, Leavis PC (1990) Calcium-induced movement of troponin I relative to actin in skeletal muscle thin filaments. Science 2: 1339–1341
Theirfelder L, MacRae C, Watkins H, Tomfohrde J, Williams M, McKenna W, Bohm K, Noeske G, Schlepper M, Bowcock A, Vosberg H, Seidman JG, Seidman CE (1993) A familial hypertrophic cardiomyopathy locus maps to chromosome 15q2. Proc Natl Acad Sci 90: 6270–6274
Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg H, Seidman JG, Seidman CE (1994) α-Tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy; a disease of the sarcomere. Cell 77: 701–712
Tobacman LS (1988) Structure-function studies of the amino-terminal region of bovine cardiac troponin T. J Biol Chem 263: 2668–2672
Tobacman IS, Lee R (1987) Isolation and functional comparison of bovine cardiac troponin T isoforms. J Biol Chem 262: 4059–4064
Townsend PJ, Barton PJR, Yacoub MH, Farza H (1995) Molecular cloning of human cardiac troponin T isoforms: expression in developing and failing heart. J Mol Cell Cardiol 27: 2223–2236
Ueno H (1984) Local structural changes in tropomyosin detected by a trypsin-probe method. Biochemistry 23: 4791–4798
Watkins H, MacRae C, Thierfelder L, Chou YH, Frenneaux M, McKenna W, Seidman JG, Seidman CE (1993) A disease locus for familial hypertrophic cardiomyopathy maps to chromosome 1q3. Nature Genetics 3: 333–337
Watkins H, McKenna WJ, Theirfelder L, Suk HJ, Anan R, O'Donoghue A, Spirito P, Matsumori A, Moravec CS, Seidman JG, Seidman CE (1995) Mutations in the genes for cardiac troponin T and α-tropomyosin in hypertrophic cardiomyopathy. N Eng J Med 332: 1058–1064
Watkins H, Rosenzweig A, Hwang D, Levi T, McKenna W, Seidman CE, Seidman JG (1992) Characteristics and prognostic implications of myosin missense mutations in familial hypertrophic cardiomyopathy. N Engl J Med 326: 1108–14
Watkins H, Theirfelder L, Anan R, Jarcho J, Matsumori A, McKenna W, Seidman JG, Seidman CE (1993) Independent origin of identical β cardiac myosin heavy-chain mutations in hypertrophic cardiomyopathy Am J Hum Genet 53: 1180–1185
White SP, Cohen C, Phillips GN Jr (1987) Structure of co-crystals of tropomyosin and troponin. Nature 325: 826–828
Wieczorek DF, Muthuchamy M in production
Willadsen KA, Butters CA, Hill LE, Tobacman LS (1992) Effects of the amino-terminal regions of tropomyosin and troponin T on thin filament assembly. J Biol Chem 267: 23746–23752
Zhang R, Zhao J, Mandveno A, Potter JD (1995) Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Cir Res 76: 1028–1035
Zot AS, Potter JD (1987) Structural aspects of troponin-tropomyosin regulation of skeletal muscle contraction. Ann Rev Biophys Chem 16: 535–559
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Palmiter, K.A., Solaro, R.J. Molecular mechanisms regulating the myofilament response to Ca2+: Implications of mutations causal for familial hypertrophic cardiomyopathy. Basic Res Cardiol 92 (Suppl 1), 63–74 (1997). https://doi.org/10.1007/BF00794070
Issue Date:
DOI: https://doi.org/10.1007/BF00794070