Abstract
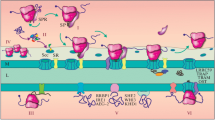
There are at least two different mechanisms for the transport of secretory proteins into the mammalian endoplasmic reticulum. Both mechanisms depend on the presence of a signal peptide on the respective precursor protein and involve a signal peptide receptor on the cis-side and signal peptidase on the trans-side of the membrane. Furthermore, both mechanisms involve a membrane component with a cytoplasmically exposed sulfhydryl. The decisive feature of the precursor protein with respect to which of the two mechanisms is used is the chain length of the polypeptide. The critical size seems to be around 70 amino acid residues (including the signal peptide). The one mechanism is used by precursor proteins larger than about 70 amino acid residues and involves two cytosolic ribonucleoparticles and their receptors on the microsomal surface. The other one is used by small precursor proteins and relies on the mature part within the precursor molecule and a cytosolic ATPase.
Similar content being viewed by others
References
Ainger, K. J., and Meyer, D. I. (1986).EMBO J. 5, 951–955.
Anderson, D. J., Mostov, K. E., and Blobel, G. (1983).Proc. Natl. Acad. Sci. USA 80, 7249–7253.
Andrews, D. W., Lauffer, L., Walter, P., and Lingappa, V. R. (1989).J. Cell. Biol. 108, 797–810.
Baker, R. K., and Lively, M. O. (1987).Biochemistry 26, 8561–8567.
Bendzko, P., Prehn, S., Pfeil, W., and Rapoport, T. A. (1982).Eur. J. Biochem. 123, 121–126.
Bernabeu, C., and Lake, J. A. (1982).Proc. Natl. Acad. Sci. USA 79, 3111–3115.
Bernstein, H. D., Poritz, M. A., Strub, K., Hoben, P. J., Brenner, S., and Walter, P. (1989).Nature 340, 482–486.
Blobel, G., and Sabatini, D. D. (1970).J. Cell. Biol. 45, 130–145.
Boman, H. G., Boman, I. A., Andreu, D., Li, Z.-qu, Merrifield, R. B., Schlenstedt, G., and Zimmermann, R. (1989).J. Biol. Chem. 264, 5852–5860.
Caulfield, M. P., Duong, L. T., and Rosenblatt, M. (1986).J. Biol. Chem. 261, 10953–10956.
Chao, C. C.-K., Bird, P., Gething, M.-J., and Sambrook, J. (1987).Mol. Cell. Biol. 7, 3842–3845.
Chirico, W. J., Waters, G. M., and Blobel, G. (1988).Nature (London)332, 805–810.
Cobet, W. W. E., Mollay, C., Müller, G., and Zimmermann, R. (1989).J. Biol. Chem. 264, 10169–10176.
Conolly, T., and Gilmore, R. (1986).J. Cell. Biol. 103, 2253–2261.
Conolly, T., and Gilmore, R. (1989).Cell 57, 599–610.
Deshaies, R. J., Koch, B. D., Werner-Washburne, M., Craig, E. A., and Schekman, R. (1988).Nature (London)332 800–805.
Garcia, P. D., and Walter, P. (1988).J. Cell. Biol. 106, 1043–1048.
Geller, B. L., and Wickner, W. (1985).J. Biol. Chem. 260, 13281–13285.
Greenburg, G., Shelness, G. S., and Blobel, G. (1989).J. Biol. Chem. 264, 15762–15765.
Hansen, W., Garcia, P. D., and Walter, P. (1986).Cell 45, 397–406.
Hortsch, M., and Meyer, D. I. (1988).Biochem. Biophys. Res. Commun. 150, 111–117.
Hortsch, M., Avossa, D., and Meyer, D. I. (1986).J. Cell. Biol. 103, 241–253.
Hull, J. D., Gilmore, R., and Lamb, R. A. (1988).J. Cell. Biol. 106, 1489–1498.
Ibrahimi, I. (1987).J. Cell. Biol. 105, 1555–1560.
Jackson, R. C., and Blobel, G. (1977).Proc. Natl. Acad. Sci. USA 74, 5598–5602.
Krieg, U. C., Walter, P., and Johnson, A. E. (1986).Proc. Natl. Acad. Sci. USA 83, 8604–8608.
Krieg, U. C., Johnson, A. E., and Walter, P. (1989).J. Cell Biol. 109 2033–2043.
Kurzchalia, T. V., Wiedmann, M., Girshovoch, A. S., Bochkareva, E. S., Bielka, H., and Rapoport, T. A. (1986).Nature (London)320, 634–636.
Lauffer, L., Garcia, P. D., Harkins, R. N., Coussens, L., Ullrich, A., and Walter, P. (1985).Nature (London)318, 334–338.
Malkin, L. I., and Richa, A. (1967).J. Mol. Biol. 26, 329–346.
Meyer, D. I., and Dobberstein, B. (1980a).J. Cell. Biol. 87, 498–502.
Meyer, D. I., and Dobberstein, B. (1980b).J. Cell. Biol. 87, 503–508.
Meyer, D. I., Krause, E., and Dobberstein, B. (1982).Nature (London)297, 647–650.
Müller, G., and Zimmermann, R. (1987).EMBO J. 6, 2099–2107.
Müller, G., and Zimmermann, R., (1988a).EMBO J. 7, 639–648.
Müller, G., and Zimmermann, R., (1988b). InGene Expression and Regulation (Bissel, M.,et al., eds.), Elsevier/North-Holland, Amsterdam, pp. 199–208.
Nicchitta, C. V., and Blobel, G. (1989).J. Cell. Biol. 108, 789–795.
Ohno-Iwashita, Y., and Wickner, W. (1983).J. Biol. Chem. 258, 1895–1900.
Ohno-Iwashita, Y., Wolfe, P., Ito, K., and Wickner, W. (1984).Biochemistry 32, 6178–6184.
Okada, Y., Frey, A. B., Guenthner, T. M., Oesch, F., Sabatini, D., and Kreibich, G. (1982).Eur. J. Biochem. 122, 393–402.
Perara, E., Rothman, R. E., and Lingappa, V. R. (1986).Science 232, 348–352.
Perlman, D., and Halvorson, H. O. (1983).J. Mol. Biol. 167, 391–409.
Rapoport, T. A., Heinrich, R., Walter, P., and Schulmeister, T. (1987).J. Mol. Biol. 195, 621–636.
Robinson, A., Kaderbhai, M. A., and Austen, B. M. (1987).Biochem. J. 242, 767–777.
Römisch, K., Webb, J., Herz, J., Prehn, S., Frank, R., Vingron, M., and Dobberstein, B. (1989)Nature (London)340, 478–482.
Roitsch, T., and Lehle, L. (1988).Eur. J. Biochem. 174, 699–705.
Rothblatt, J. A., and Meyer, D. I. (1986).EMBO J. 5, 1031–1036.
Rothblatt, J. A., Webb, J. R., Ammerer, G., and Meyer, D. I. (1987)EMBO J. 6, 3455–3464.
Sagstetter, M., and Zimmermann, R. (1988).Biochem. Biophys. Res. Commun. 153, 498–501.
Schlenstedt, G., and Zimmermann, R. (1987).EMBO J. 6, 699–703.
Schlenstedt, G., Wachter, E., Sagstetter, M., Morel, F., Zimmermann, R., Gudmundsson, G. H., and Boman, H. G. (1989). InDynamics and Biogenesis of Membranes (Op den Kamp, J. A. F., ed.), Springer, Berlin/Heidelberg, pp. 311–326.
Schlenstedt, G., Gudmundsson, G. H., Boman, H. G., and Zimmermann, R. (1990).J. Biol. Chem. 265, 13960–13968.
Shelness, G. S., Kanwar, Y. S., and Blobel, G. (1988).J. Biol. Chem. 263, 17063–17070.
Siegel, V., and Walter, P. (1988).Cell. 52, 39–49.
Tajima, S., Lauffer, L., Rath, V. L., and Walter, P. (1986).J. Cell. Biol. 103, 1167–1178.
von Heijne, G. (1981)Eur. J. Biochem. 116, 419–422.
von Heijne, G. (1984).EMBO J. 3, 2315–2318.
Walter, P., and Blobel, G. (1981a).J. Cell Biol. 91, 551–556.
Walter, P., and Blobel, G. (1981b).J. Cell Biol. 91, 557–561.
Walter, P., Ibrahimi, I., and Blobel, G. (1981).J. Cell. Biol. 91, 545–550.
Waters, M. G., and Blobel, G. (1986).J. Cell Biol. 102, 1543–1550.
Waters, M. G., Chirico, W. J., and Blobel, G. (1986).J. Cell. Biol. 103, 2629–2636.
Watts, C., Silver, P., and Wickner, W. (1981).Cell 25, 347–353.
Watts, C., Wickner, W., and Zimmermann, R. (1983).Proc. Natl. Acad. Sci. USA 80, 2809–2813.
Wiech, H., Sagstetter, M., Müller, G., and Zimmermann, R. (1987).EMBO J. 6, 1011–1016.
Wiedmann, M., Kurzchalia, T. V., Hartmann, E., and Rapoport, T. A. (1987a).Nature (London)328, 830–833.
Wiedmann, M., Kurzchalia, T. V., Bielka, H., and Rapoport, T. A. (1987b).J. Cell Biol. 104, 201–208.
Zimmermann, R., and Wickner, W. (1983).J. Biol. Chem. 258, 3920–3925.
Zimmermann, R., and Mollay, C. (1986).J. Biol. Chem. 261, 12889–12895.
Zimmermann, R., Sagstetter, M. Lewis, J. L., and Pelham, H. R. B. (1988a).EMBO J. 7, 2875–2880.
Zimmermann, R., Sagstetter, M., Schlenstedt, G., Wiech, H., Kaßeckert, B., and Müller, G. (1988b). inMembrane Biogenesis (Op den Kamp, J. A. F., ed.), Springer, Berlin/Heidelberg, pp. 337–350.
Zimmermann, R., Sagstetter, M., and Schlenstedt, G. (1990).Biochimie 72, 95–101.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zimmermann, R., Zimmermann, M., Wiech, H. et al. Ribonucleoparticle-independent transport of proteins into mammalian microsomes. J Bioenerg Biomembr 22, 711–723 (1990). https://doi.org/10.1007/BF00786927
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00786927