Summary
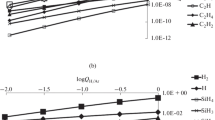
A thermodynamic calculation of equilibria in the system carbide-oxygen was carried out. It is shown that, in the presence of oxygen, the thermodynamic stability of carbides decreases sharply, i.e., carbides react with oxygen with the formation of corresponding gaseous products.
In a nitrogen atmosphere, the reactions of nitrogen with titanium and vanadium carbides are possible at relatively low temperatures, and result in the formation of the corresponding nitrides and carbon. The pressure of the gaseous products of the carbide oxidation reaction was calculated, and the equations of its temperature dependence were derived.
Similar content being viewed by others
Literature cited
A. I. Brodskii, Physical Chemistry [in Russian], Goskhimizdat 1 (1948).
J. O'M. Bockris, J. L. White, and J. D. Mackenzie, Physicochemical Measurements at High Temperatures, London, 353 (1959).
Author information
Authors and Affiliations
Additional information
Translated from Poroshkovaya Metallurgiya, No. 4 (52), pp. 46–52, April, 1967.
Rights and permissions
About this article
Cite this article
Voitovich, R.F., Shakhanova, N.P. Thermodynamic calculation of equilibria in the system carbide-oxygen. Powder Metall Met Ceram 6, 291–296 (1967). https://doi.org/10.1007/BF00775847
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00775847