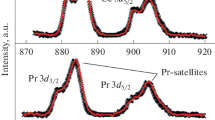
Abstract
X-ray photoelectron spectra of Te 3d5/2 and Mo 3d5/2 core electrons of TeO2-MoO3 provided an evidence for reduction of Te to the metallic state even in the presence of oxygen in the feed, while the component Mo was rather stable under reductive environment, in the vaporphase oxidation of ethyl lactate at 300 °C. Lattice oxygen was supplied to make up for the oxygen-deficit at the surface, and the catalyst should be used under oxidative, oxygen-rich conditions.
Similar content being viewed by others
References
S. Sugiyama, N. Shigemoto, N. Masaoka, S. Suetoh, H. Kawami, K. Miyaura and H. Hayashi, Bull. Chem. Soc. Japan 66 (1993), in press.
The Merck Index of Chemicals and Drugs, 6th Ed. (Merck, Darmstadt, 1952) p. 934.
Y. Okamoto, K. Oh-hiraki, T. Imanaka and S. Teranishi, J. Catal. 71 (1981) 99.
T.A. Patterson, J.C. Carver, D.E. Leyden and D.M. Hercules, J. Phys. Chem. 80 (1976) 1700.
M.K. Bahl, R.L. Watson and K.S. Irgolic, J. Chem. Phys. 66 (1977) 5526.
W.E. Swartz Jr., K.J. Wynne and D.M. Hercules, Anal. Chem. 43 (1971) 1884.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hayashi, H., Shigemoto, N., Sugiyama, S. et al. X-ray photoelectron spectra for the oxidation state of TeO2-MoO3 catalyst in the vapor-phase selective oxidation of ethyl lactate to pyruvate. Catal Lett 19, 273–277 (1993). https://doi.org/10.1007/BF00771764
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00771764