Abstract
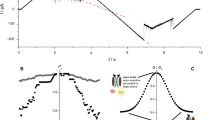
The channel-forming protein, VDAC, located in the mitochondrial outer membrane, is probably responsible for the high permeability of the outer membrane to small molecules. The ability to regulate this channelin vitro raises the possibility that VDAC may perform a regulatory rolein vivo. VDAC exists in multiple, quasi-degenerate conformations with different permeability properties. Therefore a modest input of energy can change VDAC's conformation. The ability to use a membrane potential to convert VDAC from a high (open) to a low (closed) conducting form indicates the presence of a sensor in the protein that allows it to respond to the electric field. Titration and modification experiments point to a polyvalent, positively charged sensor. Soluble, polyvalent anions such as dextran sulfate and Konig's polyanion seem to be able to interact with the sensor to induce channel closure. Thus there are multiple ways of applying a force on the sensor so as to induce a conformational change in VDAC. Perhaps cells use one or more of these methods.
Similar content being viewed by others
References
Adelesberger-Mangan, D. M. (1986). Thesis submitted to the University of Maryland at College Park.
Anderson, O. S. (1983).Biophys. J. 41, 147–165.
Bowen, K. A., Tam, K., and Colombini, M. (1985).J. Membr. Biol. 86, 51–59.
Colombini, M. (1979).Nature (London)279, 643–645.
Colombini, M. (1980).J. Membr. Biol. 53, 79–84.
Dill, E. T., Holden, M. J., and Colombini, M. (1986).J. Cell Biol. 103, 522a.
Doring, C., and Colombini, M. (1985).J. Membr. Biol. 83, 81–86.
Ehrenstein, G., Lecar, H., and Nossal, R. (1970).J. Gen. Physiol. 55, 119–133.
Freitag, H., Neupert, W., and Benz, R. (1982).Eur. J. Biochem. 123, 629–636.
Konig, T., Kocsis, B., Meszaros, L., Nahm, K., Zoltan, S., and Horvath, I. (1977).Biochim. Biophys. Acta 462, 380–389.
Konig, T., Stipani, I., Horvath, I., and Palmieri, F. (1982).J. Bioenerg. Biomembr. 14, 297–305.
Lauger, P. (1976).Biochim. Biophys. Acta 455, 493–509.
Mangan, P. S., and Colombini, M. (1987).Proc. Natl. Acad. Sci. USA,84, 4896–4900.
Mannella, C. A. (1982).J. Cell Biol. 94, 680–687.
Mannella, C. A. and Bonner, W. D., Jr. (1975).Biochim. Biophys. Acta 413, 213–225.
Mannella, C. A., and Colombini, M. (1984).Biochim. Biophys. Acta 774, 206–214.
Mannella, C. A., Colombini, M., and Frank, J. (1983).Proc. Natl. Acad. Sci. USA 80, 2243–2247.
Pfaff, E., Klingenberg, E., Ritt, E., and Vogell, W. (1968).Eur. J. Biochem. 5, 222–232.
Schein, S. J., Colombini, M., and Finkelstein, A. (1976).J. Membr. Biol. 30, 99–120.
Smack, D. P., and Colombini, M. (1985).Plant Physiol. 79, 1094–1097.
Tung, J. (1986). Thesis submitted to the University of Maryland at College Park.
Werkheiser, W. C., and Bartley, W. (1957).Biochem. J. 66, 79–91.
Wojtczak, L., and Zaluska, H. (1969).Biochim. Biophys. Acta 193, 64–72.
Yeung, C. L., Tung, J., Colombini, M., and Konig, T. (1986).Biophys. J. 49, 206a.
Zalman, L. S., Nikaido, H., and Kagawa, Y. (1980).J. Biol. Chem. 255, 1771–1774.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Colombini, M. Regulation of the mitochondrial outer membrane channel, VDAC. J Bioenerg Biomembr 19, 309–320 (1987). https://doi.org/10.1007/BF00768534
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00768534