Abstract
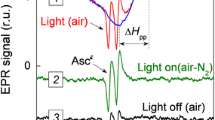
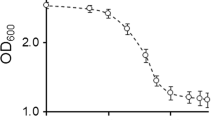
Ascorbate is stabilized in the presence of HL-60 cells. This stabilization has been questioned as a simple chemical effect. Further properties and controls about the enzymatic nature of this stabilization are described and discussed. Our results showed that cAMP derivatives and cAMP-increasing agents stimulated the ability of HL-60 cells to stabilize ascorbate. On the other hand, tunicamycin, a glycosylation-interfering agent, inhibited this ability. These data, together with hormonal regulation, support the hypothesis of an enzymatic redox system located at the plasma membrane as being responsible for the extracellular ascorbate stabilization by HL-60 cells.
Similar content being viewed by others
References
Alcaín, F. J., Burón, M. I., Rodríguez-Aguilera, J. C., Villalba, J. M., and Navas, P. (1990).Cancer Res. 50, 5887–5891.
Alcaín, F. J., Burón, M. I., Villalba, J. M., and Navas, P. (1991).Biochim. Biophys. Acta 1073, 380–405.
Alcaín, F. J., Villalba, J. M., Löw, H., Crane, F. L., and Navas, P. (1992).Biochem. Biophys. Res. Commun. 186, 951–955.
Anderson, R., Oosthuizen, R., Maritz, R., Theron, A., and van Rensbur, A. J. (1980).Am. J. Clin. Nutr. 33, 71–76.
Bielski, B. H. J., Allen, A. O., and Schwarz, H. A. (1981).J. Am. Chem. Soc. 103, 3516–3518.
Bradford, M. M. (1976).Anal. Biochem. 72, 248.
Bucttner, G. R. (1988).J. Biochem. Biophys. Methods 16, 27–40.
Burón, M. I., Rodríguez-Aguilera, J. C., Alcaín, F. J., and Navas, P. (1993).Biochem. Biophys. Res. Commun. 192, 439–445.
Chasin, M., and Harris, D. N. (1976).Cycl. Nucleotide Res. 7, 225–264.
Coassin, M., Tomasi, A., Vannini, V., and Ursini, F. (1991).Arch. Biochem. Biophys. 290, 458–462.
Crane, F. L., Sun, I. L., Clark, M. G., Grebing, C., and Löw, H. (1985).Biochim. Biophys. Acta 811, 233–264.
Crane, F. L., Löw, H., Sun, I. L., and Isaksson, M. (1990). InOxidoreduction at the Plasma Membrane: Relation to Growth and Transport (Morré, D. J., Crane, F. L., and Löw, H., eds.), CRC Press, Boca Raton, Florida, pp. 141–170.
Crowe, R. A., Taparowsky, E. J., and Crane, F. L. (1993).Biochem. Biophys. Res. Commun. 196, 844–850.
Diliberto, E. J., Jr., Dean, G., Carter, C., and Allen, P. L. (1982).J. Neurochem. 39, 563–568.
Gershoff, S. N. (1993).Nutr. Rev. 51, 313–326.
Gibson-Berry, K. L., Whitin, J. C., and Cohen, H. J. (1993).J. Neuroimmunol. 43, 59–68.
Goldenberg, H., Grebing, C., and Löw, H. (1983).Biochem. Int. 6, 1–10.
González-Reyes, J. A., Alcaín, F. J., Caler, J. A., Serrano, A., Córdoba, F., and Navas, P. (1994).Plant Sci., submitted.
Iyanagi, T., and Yamazaki, I. (1969).Biochim. Biophys. Acta 172, 370–381.
Kobayashi, K., Harada, Y., and Hayashi, K. (1991).Biochemistry 30, 8310–8315.
Lesuisse, E., Horion, B., Labbe, P., and Hilger, F. (1991).Biochim. J. 280, 545–548.
Luft, J. H. (1976).Int. Rev. Cytol. 45, 291–382.
Medina, M. A., Sánchez-Jiménez, F., Segura, J. A., and Núñez de Castro, J. (1988).Biochim. Biophys. Acta 946, 1–4.
Medina, M. A., del Castillo-Olivares, A., and Schweigerer, L. (1992).FEBS Lett. 311, 99–101.
Minetti, M., Fork, T., Soriani, M., Quaresima, V., Menditto, A., and Fenari, M. (1992).Biochem. J. 282, 459–465.
Morré, D. J., Crane, F. L., Sun, I. L., and Navas, P. (1987).Ann. N.Y. Acad. Sci. 498, 153–171.
Morré, D. J., Crane, F. L., Eriksson, L. C., Löw, H., and Morré, D. M. (1991).Biochim. Biophys. Acta 1057, 140–146.
Nakamura, M., and Ohtaki, S. (1993).Arch. Biochem. Biophys. 305, 84–90.
Navas, P., Estévez, A., Burón, M. I., Villalba, J. M., and Crane, F. L. (1988).Biochem. Biophys. Res. Commun. 154, 1029.
Navas, P., Alcaín, F. J., Burón, M. I., Rodríguez-Aguilera, J. C., Villalba, J. M., Morré, D. M., and Morré, D. J. (1992).FEBS Lett. 299, 223–226.
Nelson, S. R., Pazdermik, T. L., and Samson, F. E. (1992).Proc. West. Pharmacol. 35, 37–44.
Padh, H. (1990).Biochem. Cell Biol. 68, 1166–1173.
Park, C. M., and Kimler, B. F. (1991).Am. J. Clin. Nutr. 54, 12415–12465.
Raghoeban, M., Huisman, J. A. M., Van der Berg, W. B., and Van Ginneken, C. A. M. (1987).Life Sci. 40, 499–510.
Rodríguez-Aguilera, J. C., Navarro, F., Arroyo, A., Villalba, J. M., and Navas, P. (1993).J. Biol. Chem. 268, 26346–26349.
Rose, R. C., and Bode, A. M. (1992).Enzyme 46, 196–203.
Scheinder, W., and Standinger, M. (1965).Biochim. Biophys. Acta 96, 157–159.
Schweinzer, E., and Goldenberg, H. (1992).Eur. J. Biochem. 206, 807–812.
Schweinzer, E., Waeg, G., Esterbauer, H., and Goldenberg, H. (1993).FEBS Lett. 334, 106–108.
Sun, I. L., and Crane, F. L. (1984).Proc. Indiana Acad. Sci. 93, 267–274.
Sun, I. L., and Crane, F. L. (1990). InOxidoreduction at the Plasma Membrane: Relation to Growth and Transport. (Morré, D. J., Crane, F. L., and Löw, H., eds.), CRC Press, Boca Raton, Florida, pp. 257–280.
Sun, I. L., Crane, F. L., and Chou, J. Y. (1986a).Biochim. Biophys. Acta 886, 327–336.
Sun, I. L., Morré, D. J., Crane, F. L., Safranski, K., and Croze, E. M. (1984).Biochim. Biophys. Acta 797, 266–275.
Sun, I. L., Navas, P., Crane, F. L., Chou, J. Y., and Löw, H. (1986b).J. Bioenerg. Biomembr. 18, 471–486.
Villalba, J. M., Canalejo, A., Burón, M. I., Córdoba, F., and Navas, P. (1993a).Biochem. Biophys. Res. Commun. 192, 707–713.
Villalba, J. M., Canalejo, A., Rodríguez-Aguilera, J. C., Burón, M. I., Morré, D. J., and Navas, P. (1993b).J. Bioenerg. Biomembr. 25, 411–417.
Von Figura, K., Rey, M., Prinz, R., Voss, B., and Uldrich, K. (1979).Eur. J. Biochem. 101, 103–109.
Wang, C. S., and Alanpovic, P. (1978).J. Supramol. Struct. 9, 1–14.
Wayner, D. D. M., Burton, G. W., and Ingold, K. U. (1986).Biochim. Biophys. Acta 884, 119–123.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rodríguez-Aguilera, J.C., Navas, P. Extracellular ascorbate stabilization: Enzymatic or chemical process?. J Bioenerg Biomembr 26, 379–384 (1994). https://doi.org/10.1007/BF00762778
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00762778