Abstract
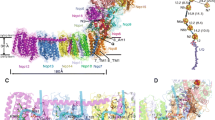
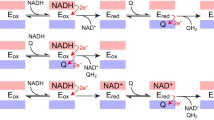
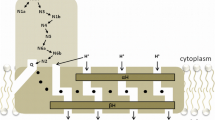
A comparison of the mitochondrial NADH-ubiquinone oxidoreductase and the energy-transducing NADH-quinone oxidoreductase (NDH-1) ofParacoccus denitrificans revealed that both systems have similar electron-transfer and energy-transduction pathways. In addition, both complexes are sensitive to the same inhibitors and contain similar electron carriers, suggesting that theParacoccus NDH-1 may serve as a useful model system for the study of the human enzyme complex. The gene cluster encoding theParacoccus NDH-1 has been cloned and sequenced. It is composed of 18,106 base pairs and contains 14 structural genes and six unidentified reading frames (URFs). The structural genes, URFs, and their polypeptides have been characterized. We also discuss nucleotide sequences which are believed to play a role in the regulation of the NDH-1 gene cluster andParacoccus NDH-1 subunits which may contain the binding sites of substrates and/or electron carriers.
Similar content being viewed by others
References
Albracht, S. P. J., van Verseveld, H. W. Hagen, W. R., and Kalkman, M. L. (1980).Biochim. Biophys. Acta 593 173–186.
Beinert, H., and Kennedy, M. C. (1989).Eur. J. Biochem. 186 5–15.
Bell, P. J., Andrews, S. C., Sivak, M. N., and Guest, J. R. (1989),J. Bacteriol. 171 3494–3503.
Bourne, R. M. and Rich, P. R. (1992).Biochem. Soc. Trans. 20 577–582.
Cammack, R. (1992).Adv. Inorg. Chem. 38 281–322.
Chen, S., and Guillory, R. J. (1984).J. Biol. Chem. 259 5124–5131.
Devereux, J., Haeberli, P., and Smithies, O. (1984).Nucleic Acids Res. 12 387–395.
Dupuis, A. (1992).FEBS Lett. 301 215–218.
Fukuyama, K., Matsubara, H., and Rogers, L. J., (1992).J. Mol. Biol. 225 775–789.
George, C. L., and Ferguson, S. J. (1987).Biochem. J. 244 661–668.
Gold, L. (1988).Annu. Rev. Biochem. 57 199–233.
Gold, L., Pribnow, D., Schneider, T., Shinedling, S., Singer, B. S., and Stormo, G. (1981).Annu. Rev. Microbiol. 35 365–403.
Hatefi, Y. (1985).Annu. Rev. Biochem. 54 1015–1069.
Hatefi, Y., Ragan, C. I., and Galante, Y. M. (1985). InThe Enzymes of Biological Membranes (Martonosi, A. N., ed.), Plenum Press, New York, pp. 1–70.
Hayashi, M., and Unemoto, T. (1987).Biochim. Biophys. Acta 890 47–54.
Hayashi, M., Miyoshi, T., Takashina, S., and Unemoto, T. (1989).Biochim. Biophys. Acta 977 62–69.
Heinrich, H. and Werner, S. (1992).Biochemistry 31 11413–11419.
Heinrich, H., Azevedo, J. E., and Werner, S. (1992).Biochemistry 31 11420–11424.
John, P., and Whatley, F. R. (1975).Nature (London) 254 495–498.
John, P., and Whatley, F. R. (1977).Biochim. Biophus. Acta 463 129–153.
Kuo, C.-F., McRee, D. E., Fisher, C. L., O'Handley, S. F., Cunningham, R. P., and Tainer, J. A. (1992).Science 258 434–440.
Kurowski, B. and Ludwig, B. (1987).J. Biol. Chem. 262 13805–13811.
Lu, P., and Rich, A. (1971).J. Mol. Biol. 58 513–531.
Matsubara, H., and Saeki, K. (1992).Adv. Inorg. Chem. 38 223–280.
Meinhardt, S. W., Kula, T., Yagi, T., Lillich, T., and Ohnishi, T. (1987a).J. Biol. Chem. 262 9147–9153.
Meinhardt, S. W., Yang, X., Trumpower, B. L., and Ohnishi, T. (1978b).J. Biol. Chem. 262 8702–8706.
Meinhardt, S. W., Matsushita, K., Kaback, H. R., and Ohnishi, T. (1989).Biochemistry 28 2153–2160.
Meinhardt, S. W., Wang, D.-C., Hon-nami, K.,Yagi, T., Oshima, T., and Ohnishi, T. (1990).J. Biol. Chem. 265 1360–1368.
Nelson, M. J., Jin, H., Turner, I. M., Grove, G., Scarrow, R. C., Brennan, B. A., and Que, L. (1991).J. Am. Chem. Soc. 113 7072–7073.
Ohnishi, T., and Salerno, J. C. (1982).Iron-Sulfur Proteins 4 285–327.
Ragan, C. I. (1987).Curr. Top. Bioenerg. 15 1–36.
Shine, J., and Dalgarno, L. (1975).Nature (London) 254 34–38.
Steinmüller, K. (1992).Plant Mol. Biol. 18 135–137.
Steinmüller, K., Ley, A. C., Steinmetz, A. A., Sayre, R. T., and Bogorad, L. (1989).Mol. Gen. Genet. 216 60–69.
Steinrücke, P., Gerhus, E., Jetzek, M., Turba, A., and Ludwig, B. (1991).J. Bioenerg. Biomembr. 23 227–239.
Stouthamer, A. H. (1992).Antonie Van Leeuwenhoek 61 1–33.
Suzuki, H., and Ozawa, T. (1986).Biochem. Biophys. Res. Commun. 138 1237–1242.
Trumpower, B. L. (1991).J. Bioenerg. Biomembr. 23 241–255.
Unemoto, T., and Hayashi, M. (1989).J. Bioenerg. Biomembr. 21 649–662.
Van der Oost, J., Haltia, T., Raitio, M., and Saraste, M. (1991).J. Bioenerg. Biomembr. 23 257–267.
Vollmer, S. J., Switzer, R. L., and Debrunner, P. G. (1983).J. Biol. Chem. 258 14284–14293.
Walker, J. E., Saraste, M., and Gay, N. J. (1984).Biochim. Biophys. Acta 768 164–200.
Walker, J. E., Arizmendi, J. M., Dupuis, A., Fearnley, I. M., Finel, M., Medd, S. M., Pilkington, S. J., Runswick, M. J., and Skehel, J. M. (1992).J. Mol. Biol. 226 1051–1072.
Weidner, U., Nehls, U., Schneider, R., Fecke, W., Leif, H., Schmiede, A., Friedrich, T., Zensen, R., Schulte, U., Ohnishi, T., and Weiss, H. (1992).Biochim. Biophys. Acta 1101 177–180.
Woods, S. A., Schwartzbach, S. D., and Guest, J. R. (1988).Biochim. Biophys. Acta 954 14–26.
Xu, X. and Yagi, T. (1991).Biochem. Biophys. Res. Commun. 174 667–672.
Xu, X., Matsuno-Yagi, A., and Yagi, T. (1991a).Biochemistry 30 6422–6428.
Xu, X., Matsuno-Yagi, A., and Yagi, T. (1991b).Biochemistry 30 8678–8684.
Xu, X., Matsuno-Yagi, A., and Yagi, T. (1992a).Arch. Biochem. Biophys. 296 40–48.
Xu, X., Matsuno-Yagi, A., and Yagi, T. (1992b).Biochemistry 31 6925–6932.
Xu, X., Matsuno-Yagi, A., and Yagi, T. (1993).Biochemistry 32 968–981.
Yagi, T. (1986).Arch. Biochem. Biophys. 250 302–311.
Yagi, T. (1987).Biochemistry 26 2822–2828.
Yagi, T. (1988).Biophysics 28 27–30.
Yagi, T. (1989).Protein Nucleic Acid Enzyme 34 351–363.
Yagi, T. (1990).Arch. Biochem. Biophys. 281 305–311.
Yagi, T. (1991).J. Bioenerg. Biomembr. 23 211–225.
Yagi, T. (1993).Biochim. Biophys. Acta 1141 1–17.
Yagi, T., and Dinh, T. M. (1990).Biochemistry 29 5515–5520.
Yagi, T., Hon-nami, K., and Ohnishi, T. (1988).Biochemistry 27 2008–2013.
Yagi, T., Xu, X., and Matsuno-Yagi, A. (1992).Biochim. Biophys. Acta 1101 181–183.
Yasunobu, K. T., and Tanaka, M. (1980).Methods Enzymol. 69 228–238.
Yumoto, N., and Tokushige, M. (1988).Biochem. Biophys. Res. Commun. 153 1236–1243.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yagi, T., Yano, T. & Matsuno-Yagi, A. Characteristics of the energy-transducing NADH-quinone oxidoreductase ofParacoccus denitrificans as revealed by biochemical, biophysical, and molecular biological approaches. J Bioenerg Biomembr 25, 339–345 (1993). https://doi.org/10.1007/BF00762459
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00762459