Abstract
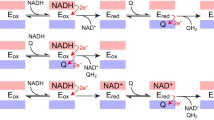
The NADH-quinone oxidoreductases of the bacterial respiratory chain could be divided in two groups depending on whether they bear an energy-coupling site. Those enzymes that bear the coupling site are designated as NADH dehydrogenase 1 (NDH-1) and those that do not as NADH dehydrogenase 2 (NDH-2). All members of the NDH-1 group analyzed to date are multiple polypeptide enzymes and contain noncovalently bound FMN and iron-sulfur clusters as prosthetic groups. The NADH-ubiquinone-1 reductase activities of NDH-1 are inhibited by rotenone, capsaicin, and dicyclohexylcarbodiimide. The NDH-2 enzymes are generally single polypeptides and contain non-covalently bound FAD and no iron-sulfur clusters. The enzymatic activities of the NDH-2 are not affected by the above inhibitors for NDH-1. Recently, it has been found that both of these types of the NADH-quinone oxidoreductase are present in a single strain of bacteria. The significance of the occurrence of these two types of enzymes in a single organism has been discussed in this review.
Similar content being viewed by others
References
Albracht, S. P. J., van Verseveld, H. W., Hagen, W. R., and Kalkman, M. L. (1980).Biochim. Biophys. Acta 593, 173–186.
Anderson, W. M., Patheja, H. S., Delinck, D. L., Baldwin, W. W., Smiley, S. T. and Chen, L. B. (1989).Biochem. Int. 19, 673–685.
Azzi, A., Casey, R. P., and Nalecz, M. J. (1984).Biochim. Biophys. Acta 768, 209–226.
Baccarini-Melandri, A., Zannoni, D., and Melandri, B. A. (1973).Biochim. Biophys. Acta 314, 298–311.
Bergsma, J., van Dongen, M. B. M., and Konings, W. N. (1982).Eur. J. Biochem. 128, 151–157.
Bragg, P. D. and Hou, C. (1967).Biochim. Biophys. Acta 119, 202–208.
Capaldi, R. A. and Vanderkooi, G. (1972).Proc. Natl. Acad. Sci. USA 69, 930–932.
Chen, S. and Guillory, R. J. (1981).J. Biol. Chem. 256, 8318–8323.
Cook, N. D., and Cammack, R., (1984).Eur. J. Biochem. 141, 573–577.
Dooijewaard, G., and Slater, E. C. (1976a).Biochim. Acta 440, 1–15.
Dooijewaard, G. and Slater, E. C. (1976b).Biochim. Biophys. Acta 440, 16–35.
Earley, F. G. P., Patel, S. D., Ragan, C. I., and Attardi, G. (1987).FEBS Lett. 219, 108–113.
Fearnley, I. M., Runswick, M. J., and Walker, J. E. (1989).EMBO J. 8, 665–672.
Galante, Y. M., and Hatefi, Y. (1979).Arch. Biochem. Biophys. 192, 559–568.
Haltia, T., Puustinen, A., and Finel, M. (1988).Eur. J. Biochem. 172, 543–546.
Haltia, T., Finel, M., Harms, N., Nakari, T., Raitio, M., Wikström, M., and Saraste, M. (1989).EMBO J. 8, 3571–3579.
Hatefi, Y. (1985).Annu. Rev. Biochem. 54, 1015–1069.
Hatefi, Y., Haavik, A. G., and Griffiths, D. E. (1962).J. Biol. Chem. 237, 1676–1680.
Hatefi, Y., Ragan, C. I. and Galante, Y. M. (1985) inThe Enzymes of Biological Membranes (Martonosi, A. N., ed.), Plenum Publishing, New York, pp. 1–70.
Hayashi, M., Miyoshi, T., Takashina, S., and Unemoto, T. (1989).Biochim. Biophys. Acta 977, 62–69.
Hendler, R. W., and Burgass, A. H. (1974).Biochim. Biophys. Acta 357, 215–230.
Hisae, N., Aizawa, K., Koyama, N., Sekiguchi, T., and Nosoh, Y. (1983).Biochim. Biophys. Acta 743, 232–238.
Hochstein, L. I. (1975).Biochim. Biophys. Acta 403, 58–66.
Hochstein, L. I., and Dalton, B. P. (1973).Biochim. Biophys. Acta 302, 216–228.
Holt, I. J., Harding, A. E., and Morgan-Hughes, J. A. (1988).Nature (London)331, 717–719.
Imagawa, T., and Nakamura, T. (1978).J. Biochem. 84, 547–557.
Imai, K., Asano, A., and Sato, R. (1968a).J. Biochem. 63, 207–218.
Imai, K., Asano, A., and Sato, R. (1968b).J. Biochem. 63, 219–225.
Ingledew, W. J., and Ohnishi, T. (1980).Biochem. J. 186, 111–117.
Jaworowski, A., Mayo, G., Shaw, D. C., Campbell, H. D. and Young, I. G. (1981).Biochemistry 20, 3621–3628.
John, P. and Whatley, F. R. (1975).Nature (London)254, 495–498.
John, P., and Whatley, F. R. (1977).Biochim. Biophys. Acta 463, 129–153.
Kadenbach, B., Kuhn-Nentwig, L., and Büge, U. (1987).Curr. Top. Bioenerg. 15, 113–161.
Kawada, N., Takeda, K., and Nosoh, Y. (1981).J. Biochem. 89, 1017–1027.
King, T. E., and Suzuki, H. (1984). InBiomedical and Clinical Aspects of Coenzyme Q (Folkers, K. and Yamamura, Y., eds.), Elsevier, Amsterdam, pp. 43.
Kita, K., Takamiya, S., Furushima, R., Ma, Y., Suzuki, H., Ozawa, T., and Oya, H. (1988).Biochim. Biophys. Acta 935, 139–140.
Krishnamoorthy, G., and Hinkle, P. C. (1988).J. Biol. Chem. 263, 17566–17575.
Leonard, K., Haiker, H., and Weiss, H. (1987).J. Mol. Biol. 194, 277–286.
Ludwig, B., and Schatz, G. (1980).Proc. Natl. Acad. Sci. USA 77, 196–200.
Mains, I., Power, P. M., and Thomas, E. W. (1980).Biochem. J. 191, 457–465.
Matsushita, K., Ohnishi, T., and Kaback, H. R. (1987).Biochemistry 26, 7732–7737.
Meijer, E. M., Wever, R., and Stouthamer, A. H. (1977).Eur. J. Biochem. 81, 267–275.
Meijer, E. M., Schuitenmaker, M. G., Boogerd, F. C., Wever, R., and Stouthamer, A. H. (1978).Arch. Microbiol. 119, 119–127.
Meinhardt, S. W., Kula, T., Yagi, T., Lillich, T., and Ohnishi, T. (1987).J. Biol. Chem. 262, 9147–9153.
Meinhardt, S. W., Matsushita, K., Kaback, H. R., and Ohnishi, T. (1989)Biochemistry 28, 2153–2160.
Meinhardt, S. W., Wang, D.-C., Hon-nami, K., Yagi, T., Oshima, T., and Ohnishi, T. (1990).J. Biol. Chem. 265, 1360–1368.
More, A. L., and Rich, P. R. (1980).Trends Biochem. Sci. TIBS 5, 284–288.
Owen, P., and Salton, M. R. J. (1975).Proc. Natl. Acad. Sci. USA 72, 3711–3715.
Owen, P., Kaback, H. R. and Graeme-Cook, K. A. (1980a).FEMS Microbiol. Lett. 7, 345–348.
Owen, P., Kaczorowski, G. J., and Kaback, H. R. (1980b).Biochemistry 19, 596–600.
Palmer, J. M., and Moller, I. M. (1982).Trends Biochem. Sci. TIBS 7, 258–261.
Phillips, M. K., and Kell, D. B. (1982).FEBS Lett. 104, 248–250.
Pilkington, S. J., and Walker, J. E. (1989).Biochemistry 19, 3257–3264.
Ragan, C. I. (1987).Curr. Top. Bioenerg. 15, 1–36.
Ramsay, R. R., Mehlhorn, R. J. and Singer, T. P. (1989a).Biochem. Biophys. Res. Commun. 159, 983–990.
Ramsay, R. R., Youngster, S. K., Nicklas, W. J., McKeown, K. A., Jin, Y.-Z., Heikkila, R. E., and Singer, T. P. (1989b).Proc. Natl. Acad. Sci. USA 86, 9168–9172.
Runswick, M. J., Gennis, R. B., Fearnley, I. M., and Walker, J. E. (1989).Biochemistry 28, 9452–9459.
Scholte, H. R. (1988).J. Bioenerg. Biomembr. 20, 161–191.
Shimomura, Y., Kawada, T., and Suzuki, M. (1989).Arch. Biochem. Biophys. 270, 573–577.
Shoffner, J. M., Lott, M. T., Voljavec, A. S., Soueidan, S. A., Costigan, D. A., and Wallace, D. C. (1989).Proc. Natl. Acad. Sci. USA 86, 7952–7956.
Stouthamer, A. H. (1980).Trends Biochem. Sci. TIBS 5, 164–166.
Unemoto, T., and Hayashi, M. (1989).J. Bioenerg. Biomembr. 21, 649–662.
de Vries, S., and Grivell, L. A. (1988).Eur. J. Biochem. 176, 377–384.
Wakao, H., Wakagi, T., and Oshima, T. (1987).J. Biochem. 102, 255–262.
Wallace, D. C., Singh, G., Lott, M. T., Hodge, J. A., Schurr, T. G., Lezza, A. M. S., Elsas, L. J., and Nikoskelainen, E. K. (1988).Science 242, 1427–1430.
Walsh, K. A. J., Daniel, R. M. and Morgan, H. W. (1983).Biochem. J. 209, 427–433.
Weiss, H. (1987).Curr. Top. Bioenerg. 15, 67–90.
Wierenga, R. K., Terpstra, P., and Hol, W. G. (1986).J. Mol. Biol. 187, 101–107.
Xu, X., Hisae, N., Koyama, N., and Nosoh, Y. (1985).FEBS Lett. 181, 313–317.
Xu, X., Kanaya, S., Koyama, N., Sekiguchi, T., Nosoh, Y., Ohashi, S., and Tsuda, K. (1989).J. Biochem. 105, 626–632.
Xu, X., Koyama, N., Cui, M., Yamagishi, A., Nosoh, Y., and Oshima, T. (1991).J. Biochem., in press.
Yagi, T. (1986).Arch. Biochem. Biophys. 250, 302–311.
Yagi, T. (1987).Biochemistry 26, 2822–2828.
Yagi, T. (1988).Biophysics 28, 27–30.
Yagi, T. (1989).Protein Nucleic Acid Enzyme 34, 351–363.
Yagi, T. (1990).Arch. Biochem. Biophys.,281, 305–311.
Yagi, T., and Dinh, T. M. (1990).Biochemistry 29, 5515–5520.
Yagi, T., and Hatefi, Y. (1988).J. Biol. Chem. 263, 16150–16155.
Yagi, T., Hon-nami, K., and Ohnishi, T. (1988).Biochemistry 27, 2008–2013.
Yang, X., and Trumpower, B. L. (1986).J. Biol. Chem. 261, 12282–12289.
Young, I. G., Rogers, B. L., Campbell, H. D., Jaworowski, A., and Shaw, D. C. (1981).Eur. J. Biochem. 116, 165–170.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yagi, T. Bacterial NADH-quinone oxidoreductases. J Bioenerg Biomembr 23, 211–225 (1991). https://doi.org/10.1007/BF00762218
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00762218