Abstract
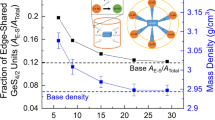
Glasses that contain at least 60 mol% GeO2 were prepared in the Bi2O3 · GeO2 and Bi2O3 · Sb2O3 · GeO2 systems. Their densities, refractive indices, and infra-red spectra were recorded. Negative molar volume deviations and positive refraction deviations occur for all of the binary glasses. These create deviations for the 60 to 80 mol % GeO2 ternary glasses that indicate non-ideal mixing when Sb3+ substitutes for Bi3+. Also, the main Ge-O stretching vibration shifts to as low as 695 cm−1 for the Bi2O3-rich binary and ternary glasses. All of these findings show that Bi2O3 more effectively depolymerizes GeO2 than does Sb2O3. The probable structural reasons for this behaviour are discussed.
Similar content being viewed by others
References
E. F. Riebling,J. Amer. Ceram. Soc. 56 (1973) 303.
G. Malmros,Acta Chem. Scand. 24 (1970) 384.
G. Gattow andH. Schröder,Z. Anorg. Allg. Chemie 318 (1962) 176.
G. Gattow andD. Schütze,ibid 328 (1964) 44.
E. I. Speranskya andA. A. Arshakuni,Russ. J. Inorg. Chem. 9 (1964) 226.
S. C. Abrahams, P. B. Jamieson andJ. L. Bernstein,J. Chem. Phys. 47 (1967) 4034.
R. Nitsche,J. Appl. Phys. 36 (1965) 2358.
G. Gattow andH. Fricke,Z. Anorg. Allg. Chemie 324 (1963) 287.
A. Durif,Anal. Chem. 30 (1958) 1161.
Idem, Compt. Rend. Acad. Sci. Paris 244 (1957) 2815.
A. Bishay andC. Maghrabi,Phys. Chem. Glasses 10 (1969) 1.
E. F. Riebling,J. Mater. Sci. 7 (1972) 40.
E. F. Riebling andV. Kotian,ibid 8 (1973) 1145.
R. C. Feast, “CRC Handbook of Chemistry and Physics”, 54th edition (Chemical Rubber Co, Cleveland, 1973/74) p. B-73.
E. F. Riebling,J. Chem. Phys. 55 (1971) 804.
J. Liebertz,J. Crystal Growth 5 (1969) 150.
W. C. Schumb andE. S. Rittner,J. Amer. Chem. Soc. 65 (1943) 1055.
E. Kordes,Z. Physik. Chem. B43 (1939) 173.
D. J. Segal, R. P. Santoro andR. E. Newnham,Z. Krist. 123 (1966) 73.
B. Aurivillius, C-I. Lindblom andP. Stenson,Acta Chem. Scand. 18 (1964) 1555.
G. A. Novak andG. V. Gibbs,Amer. Mineralogist 56 (1971) 791.
J. D. Mackenzie,J. Amer. Ceram. Soc. 46 (1963) 461.
A. A. Ballman,J. Crystal Growth 1 (1967) 37.
P. Tarte,Spectrochim. Acta 23A (1967) 2127.
R. Hubin andP. Tarte,ibid 27A (1971) 683.
P. Parte andJ. Preudhomme,ibid 26A (1970) 2207.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Riebling, E.F. Depolymerization of GeO2 and GeO2 · Sb2O3 glasses by Bi2O3 . J Mater Sci 9, 753–760 (1974). https://doi.org/10.1007/BF00761795
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00761795