Abstract
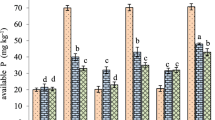
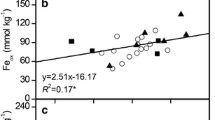
An earlier study of phosphate sorption by some savanna soils from Nigeria suggested that increased P sorption when pH was raised might be due to precipitation of exchangeable Al as amorphous polymeric Al species with increased sorption sites. But these savanna soils have Ca as the dominant cation in their exchange sites, and low exchangeable Al. The objective of this study was to determine the role played by Ca in pH-induced P sorption of three savanna soils under continuous cultivation. Phosphorus sorption increased when pH was raised from 4.5 to 7.0. Similarly, Ca retention increased with increasing pH. Regression of P sorption on Ca retention indicated a significant linear relationship in the three soils. Three possible mechanisms were proposed to explain the increasing P sorption with increasing pH: precipitation of Ca-phosphates, Ca-induced P sorption or co-adsorption of Ca and H2PO −4 or HPO 2−4 as ion pairs or complexes. Available evidence suggests that all three mechanisms can operate together to enhance P retention as pH increases. The paper proposes that increased P sorption by savanna soils when pH is raised is likely to be related to the chemistry and retention of Ca rather than to hydrolytic reactions of Al.
Similar content being viewed by others
References
Akinremi O O & Cho C M (1991) Phosphate transport in calcium saturated systems. 11. Experimental results in a model system. Soil Sci. Soc. Am. J. 55: 1282–1287
Amarasiri S L & Olsen S R (1973) Liming as related to solubility of phosphorus plant growth in acid tropical soil. Soil Sci. Soc. Am. Proc. 37: 716–721
Anderson G, Williams E & Moir J O (1974) A comparison of the sorption of inorganic orthophosphate and inositol phosphate by six acid soils. J. Soil Sci. 25: 51–62
Bolan N S, Syers J K & Sumner M E (1993) Calcium-induced sulphate adsorption by soil. Soil Sci. Soc. Am. J. 57: 691–696
Griffoen J & Appelo C A J (1993) Adsorption of calcium and its complexes by two sediments in calcium-hydrogen-chlorine-carbon dioxide system. Soil Sci. Soc. Am. J. 57: 716–712
Haynes R J (1983) Effect of lime and phosphate application on the adsorption of phosphate, sulphate and molybdate by a Spodosol. Soil Sci. 135: 221–227
Haynes R J (1984) Lime and phosphate potentials in soils. Adv. Agron. 37: 249–317
Haynes R J & Swift R S (1985) Effect of liming and air-drying on adsorption of phosphate by some acid soils. J. Soil Sci. 36: 513–521
Heathcote R G (1972) The effect of potassium and trace elements on yield in northern Nigeria. Afr. Soils 17: 85–89
Heathcote R G & Fowler A M (1977) Molybdenum deficiency in northern Nigeria. Samaru Miscellaneous Paper. Institute for Agricultural Research, Ahmadu Bello University, Zara, Nigeria
Huang C P & Stumm W (1973) Specific adsorption of cations on hydrous Al2O3. J. Colloid. Interface Sci. 43: 409–420
Jones M J & Wild A (1975) Solls of the West African Savanna. Tech. Comm. No. 55. Commonwealth Bureau of Soils, Harpenden, UK
Klinkenberg K & Higgins G M (1968) An outline of northern Nigerian savanna soils. Nig. J. Sci. 2: 91–115
Kowal J M & Kassam A H (1978) Agricultural ecology of savanna. A study of West Africa. Clarendon Press, Oxford, UK
Lindsay W L & Vlek P L (1977) Phosphate minerals.In: Dixon J B and Weed S B (eds.) Minerals in Soil Environment: 639–672. ASA, SSSA, Madison, WI, USA
Mokwunye U (1975) The influence of pH on the adsorption of phosphate by soils from the Guinea and Sudan savannah zones of Nigeria. Soil Sci. Soc. Am. Proc. 39: 1100–1102
Muljadi A, Posner A M & Quirk J P (1966) The mechanism of phosphate adsorption by kaolinite, gibbsite and pseudoboehmite. 1. The isotherms and the effect of pH on adsorption. J. Soil Sci. 17: 212–229
Murphy J & Riley J P (1962) A modified single solution for the determination of phosphorus in natural waters. Anal. Chim. Acta: 31–36
Murrmann R P & Peech M (1969) Effect of pH on labile and soluble phosphate in soils. Soil Sci. Soc. Am. Proc. 33: 205–210
Parfitt R L (1978) Anion adsorption by soils and soil materials. Adv. Agron. 30: 1–50
Pearson R G (1963) Hard and soft acids and bases. J. Am. Chem. Soc. 85: 3533–3539
Ryden J C & Syers J K (1976) Caicium retention in response to phosphate retention by soils. Soil Sci. Soc. Am. J. 40: 845–846
Sanchez P A (1976) Properties and management of soils in the tropics. A Wiley Interscience Publication, John Wiley and Sons, New York, USA
Smillie G W, Curtin D & Syers J K (1987) Influence of exchangeable calcium on phosphate retention by weakly acid soils. Soil Sci. Soc. Am. J. 51: 1169–1172
Smyth J T & Sanchez P A (1980) Effect of lime, silicate and phosphorus applications to an Oxisol on phosphorus sorption and ion retention. Soil Sci. Soc. Am. J. 44: 500–505
Sposito G & Coves J (1988) SOILCHEM: A computer program for the calculation of chemical equilibria in soil solutions and other natural water systems. Kearney foundation of soil science, University of California, Berkeley / Riverside, USA
Sposito G, Holtzclaw K M, Charlet L, Jouarny C & Page A L (1983) Sodium-calcium and sodium-magnesium exchange on Wyoming bentonite in perchlorate and chloride background ionic media. Soil Sci. Soc. Am. J. 47: 51–56
Sullivan P J (1977) The principle of hard and soft acids and bases as applied to exchangeable cation selectivity in soils. Soil Sci. 124: 117–121
Syers J K, Brownman M G, Smillie G W & Corey R B (1973) Phosphate sorption by soils evaluated by the Langmuir adsorption equation. Soil Sci. Soc. Am. Proc. 37: 358–363
Valette J A & Ibanga I J (1984) The detailed soil survey of the experimental farm of the Institute for Agricultural Research Farm, Samaru, Zaria, Nigeria. Soil Survey Bull I A R, Ahmadu Bello University, Zaria, Nigeria
Xu S & Harsh J B (1990) Hard and soft acid-base model verified for monovalent cation selectivity. Soil Sci. Soc. Am. J. 54: 1596–1601
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Agbenin, J.O. Phosphorus sorption by three cultivated savanna alfisols as influenced by pH. Fertilizer Research 44, 107–112 (1995). https://doi.org/10.1007/BF00750799
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00750799