Abstract
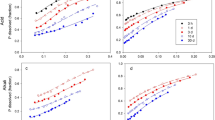
Relationships between plant response and rates of dissolution of ground (< 150µm) North Carolina phosphate rock (NCPR), NCPR 30% acidulated with phosphoric acid (NCPAPR) and monocalcium phosphate (MCP) were assessed in pot experiments. The three fertilizers were incubated for 1, 50 and 111 days, at the rates of 75, 150 and 750µg P g−1 soil, using two soils with different P-retention capacity. After each period of incubation, four pots were set up from each treatment, and perennial ryegrass (Lolium perenne) was grown in a growth chamber for about six weeks to assess the agronomic effectiveness of the fertilizers. Results in dry matter yield and P uptake showed that immediately following application (1 day incubation), the MCP (solution) was supplying more P to plants than either the NCPR or the NCPAPR applied at the same rate. After 50 and 111 days of incubation, the NCPR and NCPAPR were just as effective in the lower P-retention Tekapo soil. The relative agronomic effectiveness (RAE) of the NCPR and NCPAPR compared with MCP was generally poorer in the higher P-retention Craigieburn soil than in the Tekapo soil shortly after application, but improved with time of incubation. Ryegrass responses to the application of the three fertilizers corresponded to the changing trends of exchangeable P in the soils, measured by the isotopic method.
Regressions were made between plant P uptake and indices describing the intensity factor (water extractable P), quantity factor (Bray I P, Olsen P, 0.5M NaOH extractable P and isotopic exchangeable P) and the kinetic factor (Fin) of soil P supply to plants in the Tekapo soil. The percentage of variation in plant P uptake explained by individual indices was generally less than 80%, no matter which of the three single variable models, the Mitscherlich, the quadratic or the power function was fitted. However, more than 96% of the variation in plant P uptake in the Tekapo soil could be explained by the power function models involving two variables. The rate of P dissolution (Fin) determined by the isotopic dilution method was included in all the two variable models. The results suggest that assessment of soil P supply to plants should consider the kinetic factor in addition to the intensity and quantity factors, particularly where P fertilizers with differing solubility are applied.
Similar content being viewed by others
References
Bolland MDA, Gilkes RJ and Allen DG (1988) The residual value of superphosphate and rock phosphates for lateritic soils and its evaluation using three soil phosphate tests. Fert Res 15: 253–280
Bray RH and Kurtz LT (1945) Determination of total, organic and available forms of phosphorus in soils. Soil Sci 59: 39–45
Di HJ, Harrison R and Campbell AS (1994a) Assessment of methods for studying the dissolution of phosphate fertilizers of differing solubility in soil. I. An isotopic method. Fert Res (This issue)
Di HJ, Harrison R and Campbell AS (1994b) Assessment of methods for studying the dissolution of phosphate fertilizers of differing solubility in soil. II. Chemical extractions and comparison with isotopic exchange. Fert Res (This issue)
Draper NR and Smith H (1981) Applied Regression Analysis. 2nd ed. John Wiley and Sons Inc., New York. 709 p
Gunary D and Sutton CD (1967) Soil factors affecting plant uptake of phosphate. J Soil Sci 18: 167–173
Hammond LL, Chien SA and Mokwunye AU (1986) Agronomic value of unacidulated and partially acidulated phosphate rocks indigenous to the tropics. Adv Agron 40: 89–140
Holford ICR (1991) Comments on intensity-quantity aspects of soil phosphorus. Aust J Soil Res 29: 11–14
Larsen S (1952) The use of P32 in studies on the uptake of phosphorus by plants. Plant Soil 4: 1–10
Larsen S (1967) Soil phosphorus. Adv Agron 19: 151–210
Olsen SR and Sommers LE (1982) Phosphorus. In: AL Page (ed) Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties. 2nd ed, pp 403–430. Am Soc Agron, Madison, Wisconsin, USA
Olsen SR, Cole CV, Watanabe FS and Dean L (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular 939
Russell RS, Rickson JB and Adams SN (1954) Isotope equilibria between phosphates in soil and their signifcance in the assessment of fertility by tracer methods. J Soil Sci 5: 85–105
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Di, H.J., Harrison, R. & Campbell, A.S. Assessment of methods for studying the dissolution of phosphate fertilizers of differing solubility in soil. Fertilizer Research 38, 19–27 (1994). https://doi.org/10.1007/BF00750059
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00750059