Abstract
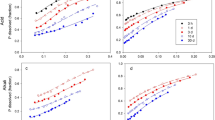
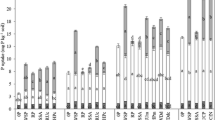
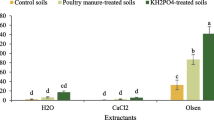
The dissolution of three phosphate fertilizers, ground (<150µm) North Carolina phosphate rock (NCPR), NCPR 30% acidulated with phosphoric acid (NCPAPR), and monocalcium phosphate (MCP) were studied using six chemical extraction methods, 0.5M NaOH followed by 1M HCl extractable P, 0.5M BaCl2 (buffered at pH = 8.1 with triethanolamine, BaCl2/TEA) extractable Ca, Olsen P, Bray I P and water extractable P. Two soils were used, Tekapo fine sandy loam and Craigieburn silt loam. Extractions were made after the fertilizers were incubated with the soils at the rates of 75,150 and 750µg P g−1 soil for 1, 8, 24, 51 and 111 days. Percentage dissolution of the PR-containing fertilizers was found to differ significantly between the extractants, 0.5M NaOH, 1M HCl and 0.5M BaCl2/TEA. These differences in estimated dissolution rates between methods were attributed to differences in the recovery rates of P or Ca between methods, which depended on the type of the extractant, soil P-retention capacity or Ca-saturation, and on the fertilizer application rate. No one method was found to be clearly better than others in studying PR dissolution in soils. The 0.5M NaOH extractable P was poorly related to water extractable P (R2 = 0.55 and 0.13 in Tekapo and Craigieburn soils respectively), Olsen P (R2 = 0.88 and 0.78) and Bray I P (R2= 0.88 and 0.78).
The average rates of PR dissolution measured by the isotopic method (Fin) were higher than those measured by 0.5M NaOH, 1M HCl and 0.5M BaCl2/TEA methods for the periods of 1–50 and 50–111 days of soil-fertilizer contact. The descrepancy was attributed to a plant effect on PR dissolution and to a recycling effect of fertilizer P (i.e. fertilizer P which had been transformed to slowly exchangeable forms, during incubation, fluxing back to exchangeable P pool, during the labelling trial) both accounted for by Fin values but not by extraction-derived values. The changes of water extractable P, Bray I P and Olsen P with incubation time were in agreement with those predicted by Fin and Fout values. This suggests that Fin and Fout are two important parameters indicating the rates of phosphate release to and retention from plant available P pool.
Similar content being viewed by others
References
Blakemore LC, Searle PL and Daly BK (1987) Methods for chemical analysis of soils. NZ Soil Bur Sci Report 80: 31–45
Bolan NS and Hedley MJ (1989) Dissolution of phosphate rocks in soils.1. Evaluation of extraction methods for the measurement of phosphate rock dissolution. Fert Res 19: 65–75
Bolan NS and Hedley MJ (1990) Dissolution of phosphate rocks in soils. 2. Effect of pH on the dissolution and plant availability of phosphate rock in soil with pH dependent charge. Fert Res 24:125–134
Bray RH and Kurtz LT (1945) Determination of total, organic and available forms of phosphorus in soils. Soil Sci 59: 39–45
Di HJ, Harrison R and Campbell AS (1994) Assessment of methods for studying the dissolution of phosphate fertilizers of differing solubility in soil. I. An isotopic method. Fert Res (This issue)
Hughes JC and Gilkes RJ (1984) The effect of chemical extractant on the estimation of rock phosphate fertilizer dissolution. Aust J Soil Res 22: 475–481
Kirk GJD and Nye PH (1986) A simple model for predicting the rates of dissolution of sparingly soluble calcium phosphates. II. Application of the model. J Soil Sci 37:.541–554
Kucey RMN, Janzen HH and Leggett ME (1989) Microbially mediated increases in plant-available phosphorus. Adv Agron 42:199–228
Mackay AD, Syers JK, Tillman RW and Gregg PEH (1986) A simple model to describe the dissolution of phosphate rock in soils. Soil Sci Soc Am J 50: 291–296
Murphy J and Riley JP (1962) A modifed single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27: 31–36
Olsen SR and Sommers LE (1982) Phosphorus. In: AL Page (ed) Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties. 2nd ed, pp 403–430. Am Soc Agron, Madison, Wisconsin, USA
Olsen SR, Cole CV, Watanabe FS and Dean L (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular 939
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Di, H.J., Harrison, R. & Campbell, A.S. Assessment of methods for studying the dissolution of phosphate fertilizers of differing solubility in soil. Fertilizer Research 38, 11–18 (1994). https://doi.org/10.1007/BF00750058
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00750058