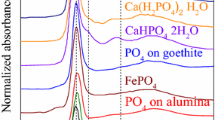
Abstract
Residual fertilizer grains were recovered using density fractionation from three Western Australian soils fertilized five years previously with North Carolina apatitic rock phosphate (RP), Queensland apatitic RP and calcined calcium-iron-aluminium RP (Calciphos). Electron micrographs, energy dispersive X-ray analyses (EDS) and unit cell parameters derived from X-ray diffraction patterns (XRD) of recovered apatitic grains do not differ from those for the original fertilizers. Dissolution of these apatitic fertilizers in soils proceeds by surface etching of apatite crystals and is congruent with no formation of intermediate P compounds. Calciphos grains also did not differ in mineralogy and composition but they had developed a porous fabric due to dissolution along subgrain boundaries. Poorly soluble superphosphate residues were mainly composed of dicalcium phosphate dihydrate, FeAl phosphate and apatite together with quartz and other soil minerals. The P concentrations in various particle size fractions of recovered fertilizer materials were related to the particle size distribution of fertilizers originally added to the soils. These results support the agronomic results that the low agronomic effectiveness of RP fertilizers in these soils is primarily due to the small extent of dissolution of RP.
Similar content being viewed by others
References
AOAC (1975) Official Methods of Analysis 12th Edn. Assoc Off Agri Chem Washington DC
Bell LC, Posner AM and Quirk JP (1973) The point of zero charge of hydroxyapatite and fluorapatite in aqueous solutions. J Colloid Interface Sci 42: 250–261
Bolland MDA and Gilkes RJ (1990) Rock phosphates are not effective fertilizers in Western Australian soils: a review of one hundred years of research. Fert Res 22: 79–95
Bolland MDA, Weatherley AJ and Gilkes RJ (1989) The longterm residual value of rock phosphate and superphosphate fertilizers for various plant species under field conditions. Fert Res 20: 89–100
Bouldin DR, Lehr JR and Sample EC (1960) The effect of associated salts on transformations of monocalcium phosphate monohydrate at the sites of application. Soil Sci Soc Am Proc 24: 464–468
Brown G and Brindley GW (1980) X-ray diffraction procedures for clay mineral identification. In: (eds) Brown G and Brindley GW pp 305–36g. Crystal structures of clay minerals and their X-ray identification, Mineralogical Society, Lonndon
Chang SC and Jackson ML (1957) Fractionation of soil phosphorus. Soil Sci 59: 133–144
Chien SH and Black CA (1975) The activity concept of phosphaterock solubility. Soil Sci Soc Am Proc 39: 856–858
Chien SH and Black CA (1976) Free energy of formation of carbonate apatites in some phosphate rocks. Soil Sci Soc Am Proc 40: 234–239
Ghosh AK and Gilkes RJ (1987) The initial and residual agronomic effectiveness of some Indian, USA and Australian rock phosphates. Fert Res 12: 201–218
Gilkes RJ and Lim-Nunez R (1980) Poorly soluble phosphates in Australian superphosphate: their nature and availability to plants. Aust J Soil Res 31: 85–95
Gilkes RJ (1975) Some properties of granulated superphosphate and its behavior in the soil. Aust J Soil Res 13: 203–15
Gilkes RJ and Palmer B (1979) Calcined Christmas Island C grade rock phosphate fertilizers: Mineralogical properties, reversion and assessment by chemical extraction. Aust J Soil Res 17: 467–481
Gillman GP and Sumpter EA (1986) Modification to the compulsive exchange method for measuring exchange characteristics of soils. Aust J Soil Res 24: 61–66
Golden DC, Stewart, Tillman RW and White RE (1991) partially acidulated reactive phosphate rock (PAPR) fertilizer and its reactions in soil. II Mineralogy and morphology of the reaction products. Fert Res 28: 295–304
Hanley, PK and Murphy MD (1984) Phosphate forms in particle size separates of Irish soils in relation to drainage and parent materials. Soil Sci Soc Am Proc 34: 587–590
Khasawneh FE and Doll EC (1978) The use of phosphate rock for direct application to soils. Adv Agron 30: 159–209
Kumar V, Gilkes RJ and Bolland MDA (1994) Forms of phosphate in Western Australian soils fertilized with rock phosphate and superphosphate as assessed by chemical fractionation. Aust J Soil. Res. (In Press)
Lehr JR (1980) Phosphate raw materials and fertilizers: part I-A look ahead. In: Khasawneh FE, Sample EC and Kamprath EJ (eds) The Role of Phosphorus in Agriculture, pp 81–120. Am, Soc. Agron. Madison, USA
Lehr JR, Brown WE, Frazier AW, Smith JP and Thrasher RD (1967) Crystallographic properties of fertilizer compounds. Chem Eng Bull No 6 Tennessee Valley Authority, Muscle Shoals, Alabama
Lindsay WL and Vlek PLG (1979) Phosphate minerals. In: Dixon J B and Weed S B (eds) Minerals in soil environments. Soil Sci. Soc. Am. Inc., Madison, WI. 639 p
Lindsay WL and Stephenson HF (1959) Nature of the reactions of monocalcium phosphate monohydrate in soils: The solution that reacts with the soil. Soil Sci Soc Am Proc 23: 12–18
McClellan GH and Gremillion LR (1980) Evaluation of phosphatic raw materials. In: Khasawneh FE, Sample EC and Kamprath EJ (eds) The Role of Phosphorus in Agriculture, pp 42–80. Am Soc Agron, Madison, USA
Mehra OP and Jackson ML (1960) Iron oxide removal from soils and clay by a dithionite-citrate system buffered with sodium bicarbonate. Clay Miner 7: 317–327
Norrish K (1968) Some phosphate minerals of soils. In: JW Holmes (ed) Trans. Int. Congr. Soil Sci., 9th, pp 713–723. Adelaide. Elsevier, New York
Northcote KH (1979) A factual key for the recognition of Australian soils.4th Ed. Rellim Tech Publ, Glenside, South Australia
Novok GA and Colville AA (1989) A practical interactive leastsquare cell parameter program using an electronic spreadsheet and a personal computer. Am Mineral 74: 488–490
Ozanne PG and Shaw TC (196T) Phosphate sorption by soils as a measure of phosphate requirement for pasture growth. Aust J Agri Res 18: 601–612
Palmer B and Gilkes RJ (1982) Reversion of calcined calcium aluminium phosphate fertilizers due to rehydroxylation of crandallite. Aust J Soil Res 20: 243–250
Pierzynski GM (1991) The chemistry and mineralogy of phosphorus in excessively fertilized soils. Critical reviews in Environment Control 21: 265–295
Pierzynski GM,Logan TJ,Traina SJ and Bigham JM (1990a) Phosphorus chemistry and mineralogy of phosphorus in excessively fertilized soils: Quantitative analysis of phosphorus-rich particles. Soil Sci Soc Am J 54: 1576–1583
Pierzynski GM, Logan TJ,Traina SJ and Bigham JM (1990b) Phosphorus chemistry and mineralogy of phosphorus in excessively fertilized soils: Descriptions of phosphorus-rich particles. Soil Sci Soc Am J 54: 1584–1589
Qureshi RH, Jenkins DA, Davies RI and Reds JA (1969) Application of microprobe analysis to the study of phosphorus in soils. Nature (London) 221: 1142–1143
Sample EC, Soper RJ and Racz GJ (1980) Reactions of phosphate fertilizers in soils. In: Khasawneh FE, Sample EC, Kamprath EJ (eds) The Role of Phosphorus in Agriculture, pp 263–310. Am Soc Agron, Madison, USA
Sawhney BL (1973) Electron microprobe analysis of phosphates in soils and sediments. Soil Sci Soc Am Proc. 37: 658–660
Singh Balbir and Gilkes RJ (1992) XPAS: An interactive computer programme for analysis of X-ray powder diffraction patterns. Powder Diffraction 7: 6–10
Smith AN, Posner AM and Quirk JP (1976) The estimation of the acid needed to obtain a saturated solution in equilibrium with solid hydroxyapatite. J Colloid Interface Sci 54: 176–183
Smith AN, Posner AM and Quirk JP (1977) A model describing the kinetics of dissolution of hydroxyapatite. J Colloid Interface Sci 62: 475–494
Smith AN, Posner AM and Quirk JP (1976) Incongruent dissolution and surface complexes of hydroxyapatite. J Colloid Interface Sci. 54: 176–183
Steward. J H and J M Oades (1972) The determination of organic phosphorus in soils. J Soil Sci 23: 38–49
Wang HD, Harris WG and Yuan TL (1989) Phosphate minerals in some Florida phosphatic soils. Soil and Crop Sci Soc Fla Proc 48: 49–55
White MS (1976) Compounds formed in the manufacture of superphosphates from the phosphates of Christmas and Nauru Islands and Queensland. N. Z J Sci 19: 421–31.
Williams JDH, Syers JK and Walker TW (1967) Fractionation of soil inorganic phosphate by a modification of Chang and Jackson's procedure. Soil Sci Soc Am Proc 31: 736–739
Wire DR, Chien SH and Black CA (1971) Solubility of hydroxyapatite. Soil Sci 111: 107–112
Yeomans JC and Bremner JM (1988) A rapid and precise method for routine determination of organic carbon in soil. Comm Soil Plant Anal 19: 1467–1476
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kumar, V., Gilkes, R.J., Armitage, T.M. et al. Identification of residual P compounds in fertilized soils using density fractionation, X-ray diffraction, scanning and transmission electron microscopy. Fertilizer Research 37, 133–149 (1994). https://doi.org/10.1007/BF00748554
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00748554