Summary
-
1.
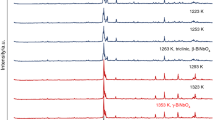
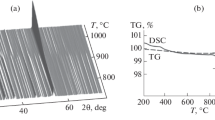
Specimens of the system BaO−WO3, heated in air at 1200o, contained the following barium tungstates: BaO·WO3, 2BaO·WO3 and 3BaO·WO3. At BaCO3: WO3=1:2 ratio in the same system a mixture of the composition BaO·WO3+WO3 is formed; this mixture melts at about 940o with formation of a compound of the probable formula BaO·2WO3.
-
2.
The main reaction product in the system BaO−WO3 is the tungstate BaO·WO3. formation of which is complete after the appropriate mixture of the original components has been heated for 2 hr at 850o. All the tungstates are stable in air for a considerable time.
-
3.
When specimens in the system BaO−MoO3 are heated in air. three compounds are likewise formed by the solid-state reaction: BaO·MoO3. 2BaO·MoO3 and 3BaO·MoO3; however, only the molybdate.BaO·MoO3 was obtained as a single phase, event at 1200o. The molybdate 2BaO·MoO3 was obtained as the single phase when a mixture of BaO·MoO3+BaCO3 was heated in air at 1200o.
-
4.
The main compound in the system BaO−MoO3 is the molybdate BaO·MoO3. The structures of the barium molybdates BaO·MoO3, 2BaO·MoO3, 3BaO·MoO3 are quite similar to the structures of the corresponding tungstates.
-
5.
When specimens of the BaO−Ta2O5 system are heated in hydrogen, five barium tantalates are formed to which the following formulas may be ascribed: 5BaO·Ta2O5, 4BaO·Ta2O5, 7BaO·3Ta2O5, BaO·Ta2O5 and 3BaO·7Ta2O5. The compounds to which the formulas 7BaO·3Ta2O5 and 3BaO·7Ta2O5 are ascribed may in fact be the compounds 2.5 BaO·Ta2O5 and BaO·2.5 Ta2O5; the mixtures corresponding to the latter compostions had not been investigated.
The results obtained for the system BaO−Ta2O5 cannot be regarded as completely reliable at this time. because the specimens were heated in hydrogen; this may distort the true phase picture somewhat although almost the same results were obtained when the system was heated in air at 1100. 1200. and 1300o.
Similar content being viewed by others
Literature cited
E. G. Steward and H. P. Rooksby, Nature157, 548 (1946).
E. P. Ostapchenko, Dissertation (Gos. soyuzn. inst. Ministerstva radiotekhnicheskoi prom., 1954).
V. A. Pchelkin, A. F. Efimov, and A. V. Lapitskii, Zh. obshch. khimii24, 1284, 1495 (1954).
A. I. Kitaigorodskii, X-Ray Structure Analysis of Microcrystalline and Amorphous Substances [in Russian] (Gostekhizdat, Moscow, 1952.
A. I. Zaslavskii, R. A. Zvinchuk, and A. G. Tutov, Dokl. AN SSSR104, 3, 409 (1955).
R. C. Hughes, P. P. Coppola, and T. H. Evans, J. Appl. Phys.23, 6, 635 (1952).
E. G. Steward and H. P. Rooksby, Acta Cryst.4, 503 (1951).
Additional information
Translated from Zhurnal Strukturnoi Khimii, Vol. 2, No. 1. pp. 33–45, January–February, 1961
Rights and permissions
About this article
Cite this article
Zhmud', E.S., Ostapchenko, E.P. X-ray investigation of the systems BaO−WO3, BaO−MoO3 and BaO−Ta2O5 . J Struct Chem 2, 27–37 (1961). https://doi.org/10.1007/BF00744852
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00744852