Abstract
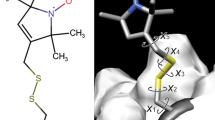
The distribution of lipid in the cytochrome oxidase-lipid complex from beef heart mitochondria has been studied by the spin labeling electron spin resonance technique. The spectra of a phospholipid spin label incorporated in the complex reveals an immobilized (on the ESR time scale) component in addition to the fluid component which is found in aqueous dispersions of the extracted lipids. The first component corresponds to the domain of lipid influenced by the protein, and the second component to the remaining lipid. A theory taking into account not only the sizes of the lipid regions in which the spin label molecule distributes itself, but also the different affinities of the label for the two domains, has been developed. Taking advantage of the variation in spectra obtained with increasing amounts of spin label, computer calculations have been performed to estimate the distribution of lipid in the different regions of the cytochrome oxidase-lipid complex. An extrapolation of the amount of immobilized spin-labeled phospholipid to zero concentration of label allows a calculation of the number of fatty acid residues interacting with the protein to be made. It has been found that the number of aliphatic chains influenced by the protein is higher than that calculated for a single boundary layer around the protein. The approach used in this paper can be useful for studies of protein-lipid interactions in other systems.
Similar content being viewed by others
References
Benga, G., and Chapman, D. (1976).Rev. Roum. Biochim. 13 251–259.
Bieri, V., Wallach, D., and Lin, P. (1974).Proc. Natl. Acad. Sci. 71 4797–4801.
Boss, W. F., Kelley, C. J., and Landsberger, F. E. (1975).Anal. Biochem. 64 289–292.
Butler, K. W., Tatrie, N. H., and Smith, I. C. P. (1974).Biochim. Biophys. Acta 363 351–360.
Fleischer, S., and Fleischer, B. (1967). InMethods in Enzymology, Vol. 10, R. W. Estabrook and M. Z. Pullman, eds., Academic Press, New York, pp. 406–433.
Griffith, O. H., Jost, P., Capaldi, R. A., and Vanderkooi, G. (1973).Ann. N.Y. Acad. Sci. 222 561–572.
Jost, P., Capaldi, R. A., Vanderkooi, G., and Griffith, O. H. (1973a).J. Supramol. Struct. 1 269–278.
Jost, P. C., Griffith, O. H., Capaldi, R. A., and Vanderkooi, G. (1973b).Proc. Natl. Acad. Sci. USA 70 480–484.
Jost, P., Griffith, O. H., Capaldi, R. A., and Vanderkooi, G. (1973c).Biochim. Biophys. Acta 311 141–152.
Jost, P. C., Nadakavukaren, K. K., and Griffith, O. H. (1977).Biochemistry 16 3110–3114.
Knowles, P. F., Watts, A., and Marsh, D. (1979).Biochemistry 18 4480–4487.
Kuboyama, M., Yong, F. C., and King, T. E. (1972).J. Biol. Chem. 247 6375–6385.
Marsh, D., and Barrantes, F. J. (1978).Proc. Natl. Acad. Sci. USA 75 4329–4333.
Marsh, D., Watts, A., Maschke, W., and Knowles, P. F. (1978).Biochem. Biophys. Res. Commun. 81 397–402.
Oldfield, E., and Chapman, D. (1972).FEBS Lett. 23 285–297.
Oldfield, E., Keough, K., and Chapman, D. (1971).FEBS Lett. 20 344–346.
Porumb, T., and Slade, E. F. (1976a).J. Magn. Reson. 22 219–224.
Porumb, T., and Slade, E. F. (1976b). University of Indiana Quantum Chemistry Exchange Program, Vol. 295.
Rice, D. M., Hsung, J. C., King, T. E., and Oldfield, E. (1979).Biochemistry 18 5885–5892.
Robinson, N. C., and Capaldi, R. A. (1977).Biochemistry 16 375–381.
Sauerheber, R. D., Gordon, L. M., Crosland, R. D., and Kuwahara, M. D. (1977).J. Membr. Biol. 31 131–169.
Seelig, A., and Seelig, J. (1978).Hoppe Seyler's Z. Physiol. Chem. 359 1747–1753.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Benga, G., Porumb, T. & Wrigglesworth, J.M. Estimation of lipid regions in a cytochrome oxidase-lipid complex using spin labeling electron spin resonance: Distribution effects on the spin label. J Bioenerg Biomembr 13, 269–283 (1981). https://doi.org/10.1007/BF00743205
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00743205