Summary
-
1.
The formation of nitrate complex compound of Pu4+ when the HNO3 concentration is increased carries a stepwise character. The formation of mainly one or another complex ion takes place in a definite region of HNO3 concentration. This does not exclude the possibility of coexistence in the given HNO3 concentration range of some complex ions; a proof of this is given by the doubling and even tripling of the absorption band in the 480 m\gm region.
-
2.
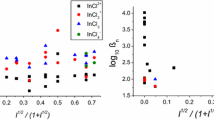
A high concentration of hydrogen ions or of cations having a significant hydration energy enhances the formation of complex ions with a high content of nitrate groups.
-
3.
The formation of nitrate complex ions of tetravalent plutonium leads to a significant shift of the absorption band. At the same time there takes place a change of the values of the molar extinction coefficients which either increase or decrease, due to the perturbations of the 5f energy levels produced by the nitrate groups replacing the water molecules in the hydrate sphere of the ion.
-
4.
The coordination number of plutonium in the formation of nitrate complex ions remains the same and equal to the number of water molecules forming the hydrate sphere of a hydrated Pu4+ ion. Since the literature data [5] give for tetravalent plutonium a coordination number equal to 8, the reaction of successive formations of nitrate complexes of plutonium may be written in the following form.
$$[Pu(H_2 O)_{8 - n} (NO_3 )_n ]^{ + 4 - n} + NO_3^ - \rightleftharpoons [Pu(H_2 O)_{8 - (n + 1)} (NO_3 )]_{n + 1}^{ + 4 - (n + 1)} + H_2 O$$The composition of the complex ions which are formed for HNO3 concentrations from 0.5 to 10–11 M is expressed by the formula [Pu(NO3)n(H2O)8−n]+4−n, where n=1,2...6.
Similar content being viewed by others
Literature cited
J. C. Hindman, Nat. Nucl. Energ. Ser., Div. IV. The Transuranium Elements (New York, 1949). Vol. 14B, Paper 4, p. 5.
S. W. Rabideau and J. F. Lemons, J. Am. Chem. Soc.73, 6, 2895 (1951).
C. K. McLane, J. S. Dixon, and J. C. Hindman, Nat. Nucl. Energ. Ser., Div. IV. The Transuranium Elements (New York, 1949). Vol. 14B, Paper 3, p. 4.
H. H. Anderson, Nat. Nucl. Energ. Ser., Div. IV. The Transuranium Elements (New York, 1949), Vol. 14B, Paper 6, p. 220.
J. C. Hindman, Nat. Nucl. Energ. Ser., Div. IV. The Transuranium Elements (New York, 1949), Vol. 14B, Paper 4, p. 7.
Additional information
Translated from Zhurnal Strukturnoi Khimii, Vol. 1, No. 2, pp. 135–144 July-August, 1960
The work was completed in 1953.
Rights and permissions
About this article
Cite this article
Lipis, L.V., Pozharskii, B.G. & Fomin, V.V. Spectrophotometric study of the complex formation. J Struct Chem 1, 125–133 (1960). https://doi.org/10.1007/BF00738928
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00738928