Conclusions
-
1.
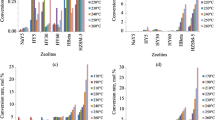
Synthetic zeolites of the NaX or CaX type possess high dynamic activity and selectivity with respect to benzene in adsorption either from the liquid or from the vapor phase, but the dynamic activity of zeolites decreases with increase in the feed rate of crude.
-
2.
NaX zeolites have a greater dynamic activity than CaX zeolites at otherwise equal conditions.
-
3.
An increase in benzene concentration in the starting material, in the limits of 4 to 20 vol.%, under unchanged layer height and with operation to breakthrough, leads to lowering of the dynamic activity of NaX zeolites.
-
4.
Carrying out desorption of benzene from NaX zeolites at 300°C and a vacuum of 730 mm Hg for 3 h allows the zeolites to be used repeatedly.
Similar content being viewed by others
Literature cited
V. A. Sokolov, N. S. Torocheshnikov, and N. V. Kel'tsev, Molecular Sieves and Their Application [in Russian], “Khimiya” Press (1964).
R. M. Barrer, British Chemical Eng., May (1959), p. 101.
Erdöl und Kohle, Erdgas petrochemie, Sept., No. 9 (1960), p. 650.
L. F. Fominykh, N. P. Smirnov, N. A. Samoilov, and L. V. Dolmatov, Khim. i Tekhnol. Topliv i Masel, No. 2 (1965).
I. I. Kipling and E. U. M. Wright, Trans. Faraday Soc.,55, No. 439 (1959), p. 1185.
Ékspress-informatsiya, Ser. Khimiya i Pererabotka Nefti, No. 32 (1959).
S. Runemann, Transactions of the Institute of Chemical Engineers,40, No. 2 (1962), p. A61.
U. S. Patent 2,818,737 (1957).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 4, pp. 8–10, April, 1967.
Rights and permissions
About this article
Cite this article
Fominykh, L.F., Samoilov, N.A., Stekol'shchikov, M.N. et al. Extraction of benzene from hydrocarbon mixtures with synthetic zeolites. Chem Technol Fuels Oils 3, 230–233 (1967). https://doi.org/10.1007/BF00731741
Issue Date:
DOI: https://doi.org/10.1007/BF00731741