Abstract
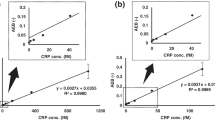
Influenza A and Sendai viruses bind toneolacto-series gangliosides isolated from human granulocytes. Differences in receptor specificity of influenza viruses A/PR/8/34 (H1N1), A/X-31 (H3N2), and parainfluenza Sendai virus (HNF1, Z-strain) were determined by two direct solid phase binding assays: the overlay technique, which combines high-resolution in the separation of gangliosides on thin-layer chromatograms with direct binding; and the microwell adsorption assay as a convenient binding assay which is performed in microtitre wells to estimate the avidity of binding to an isolated ganglioside. Both methods were applied for comparative binding studies. Viruses were found to exhibit specificity for oligosaccharides and sialic acids as well as for chain length of the neutral carbohydrate backbone, whereas differing fatty acids (C24:1 and C16:0) in the ceramide portion had no impact on virus adsorption. Terminal sialyloligosaccharides Neu5Acα2-3Galβ1-4Glc-R of GM3, and Neu5Acα2-3Galβ1-4GlcNAc-R as well as Neu5Acα2-6Galβ1-4GlcNAc-R ofneolacto-series gangliosides with nLcOse4Cer and nLcOse6Cer backbone, exhibited significant specific receptor activity towards the different viruses. To compare the data revealed from both test systems, values of virus binding were ascertained by a non-parametric statistical approach based on rank correlation. The rank correlation coefficientr s was calculated according to Spearman from each virus binding towards GM3, IV3Neu5Ac-nLcOse4Cer, IV6Neu5Ac-nLcOse4Cer and VI3Neu5Ac-nLcOse6SCer. The rank correlation coefficients 0.74, 0.95 and 0.92, which were determined for A/PR/8/34 (H1N1), A/X-31 (H3N2) and Sendai virus (HNF1, Z-strain), respectively, indicated that both assays generate highly correlated experimental data. Based on these results, analyses of virus binding on thin-layer chromatograms as well as in microwells were found equivalent tools for ganglioside receptor studies.
Similar content being viewed by others
References
Hakomori S (1981)Ann Rev Biochem 50:733–64.
Thompson TE, Tillack TW (1985)Ann Rev Biophys Biophys Chem 14:361–86.
Stults CLM, Sweeley CC, Macher BA (1989)Methods Enzymol 179:167–214.
Zeller BC, Marchase RB (1992)Am J Physiol 31:C1341–55.
Schauer R (1988)Adv Exp Med Biol 228:47–72.
Hakomori S (1984) InThe Cell Membrane (Habes E, ed.) pp. 181–201. New York: Plenum Press.
Karlsson KA (1989)Annu Rev Biochem 58:309–50.
Paulson JC (1985) InThe Receptors, Vol. II (Conn PM, ed.) pp. 131–219. Orlando: Academic Press.
Markwell MAK, Paulson JC (1980)Proc Natl Acad Sci USA 77:5693–97.
Suzuki Y, Nakao T, Ito T, Watanabe N, Toda Y, Guiyun X, Suzuki T, Kobayashi T, Kimura Y, Yamada A, Sugawara K, Nishimura H, Kitame F, Nakamura K, Deya E, Kiso M, Hasegawa A (1992)Virology 189:121–31.
Markwell MAK, Moss J, Hom BE, Fishman PH, Svennerholm L (1986)Virology 155:356–64.
Wilschut J (1991) InMembrane Fusion (Wilschut J, Hoekstra D, eds.) pp. 89–126. New York: Marcel Dekker.
Bergelson LD, Burkinskaya AD, Prokazova NV, Shaposhnikova GI, Kocharov SL, Shevchenko VP, Kornilaeva GV, Fomina-Ageeva EV (1982)Eur J Biochem 128:467–74.
Holmgren J, Elwing H, Fredman P, Svennerholm L (1980)Eur J Biochem 106:371–79.
Hansson GC, Karlsson KA, Larson G, Strömberg N, Thurin J, Örvell C, Norrby E (1984)FEBS Lett 170:15–18.
Karlsson KA, Strömberg N (1987)Methods Enzymol 138:220–32.
Müthing J, Unland F, Heitmann D, Orlich M, Hanisch FG, Peter-Katalinić J, Knäuper V, Tschesche H, Kelm S, Schauer R, Lehmann J (1993)Glycoconjugate J 10:120–26.
Folch J, Lees M, Sloane Stanley GH (1957)J Biol Chem 226:497–509.
Müthing J, Egge H, Kniep B, Mühlradt PF (1987)Eur J Biochem 163:407–16.
Ueno K, Ando S, Yu RK (1978)J Lipid Res 19:863–711.
Svennerholm L (1957)Biochim Biophys Acta 24:604–11.
Müthing J, Heitmann D (1993)Anal Biochem 208:121–24.
Orlich M, Khatchikian D, Teigler A, Rott R (1990)Virology 176:531–38.
Magnani JL, Smith DF, Ginsburg V (1980)Anal Biochem 109:399–402.
Olds EG (1938)Ann Math Statist 9:133–48.
Olds EG (1949)Ann Math Statist 20:117–18.
Siegel S, Castellan NJ (1988) InNonparametric Statistics, 2nd ed., hardcover text edition. New York: McGraw-Hill, Inc.
Paulson JC, Rogers GN (1987)Methods Enzymol 138:162–68.
Nobusawa E, Aoyama T, Kato H, Suzuki Y, Tateno Y, Nakajima K (1991)Virology 182:475–85.
Suzuki Y, Nagao Y, Kato H, Matsumoto M, Nerome K, Nakajima K, Nobusawa E (1986)J Biol Chem 36:17057–61.
Umeda M, Nojima S, Inoue K (1984)Virology 133:172–82.
Yiu SCK, Lingwood CA (1992)Anal Biochem 202:188–92.
Sakkar DP, Blumenthal R (1987)Membr Biochem 7:231–47.
Huang RTC (1983)Lipids 18:489–92.
Reuter G, Schauer R (1988)Glycoconjugate J 5:133–35.
IUPAC-IUB, Commission on Biochemical Nomenclature (1977)Eur J Biochem 79:11–21.
Svennerholm L (1963)J Neurochem 10:613–23.
Author information
Authors and Affiliations
Additional information
Abbreviations: BSA, bovine serum albumin; GSL(s), glycosphingolipids; HPTLC, high performance thin-layer chromatography; PBS, phosphate buffered saline; Neu5Ac,N-acetylneuraminic acid [35];r s = rank correlation coefficient according to Spearman. The designation of the glycosphingolipids follows the IUPAC-IUB recommendations [36]. LacCer or lactosylceramide, Galβ1-4Glcβ1-1Cer; lacto-N-neotetraosylceramide or nLcOse4Cer, Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ1-1Cer; lacto-N-norhexaosylceramide or nLcOse6Cer, Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ1-1Cer; GM3 (according to Svennerholm [37]) or II3Neu5AcLacCer.
Rights and permissions
About this article
Cite this article
Müthing, J., Unland, F. A comparative assessment of TLC overlay technique and microwell adsorption assay in the examination of influenza A and Sendai virus specificities towards oligosaccharides and sialic acid linkages of gangliosides. Glycoconjugate J 11, 486–492 (1994). https://doi.org/10.1007/BF00731285
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00731285