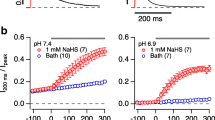
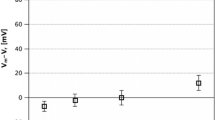
Abstract
In internally dialyzed voltage-clamped squid axons, intracellular or extracellular addition of the sulfhydryl group (-SH) specific reagentp-hydroxymercuriphenylsulfonic acid (PHMPS), causes major modifications in the magnitude and kinetic parameters of the delayed rectifier K+ current. PHMPS produces a dramatic slow-down of the macroscopic current activation kinetics with a simultaneous reduction in its amplitude. In addition, it causes a marked increase in the delay of the macroscopic current at various pre-pulse potentials (Cole-Moore shift). The main effect of PHMPS at the single channel level is a sharp decrease in the open probability (4- to 5-fold). There is, however, a small reduction in single channel conductance (20%). Gating current experiments indicate that PHMPS causes a reduction in the voltage dependence of the activation process as well as a shift of the charge/voltage relationship towards more positive potentials. This, together with an increase in the mean open time, suggests that the open state has been destabilized. The results indicate that the reaction of -SH groups with PHMPS differentially affects the gating process. All the above mentioned effects are partially reversed by either dithiotreitol orβ-mercaptoethanol,-SH group reducing agents.
Similar content being viewed by others
References
Baumgold JC, Matsumoto G, Tasaki I (1978) Biochemical studies of nerve excitability: the use of protein modifying reagents for characterizing sites involved in nerve excitation. J Neurochem 30:91–100
Bezanilla F, Armstrong CM (1977) Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol 70:549–566
Bezanilla F, Vandenberg C (1990) The cut-open axon technique. In: Gilbert DL, Adelman JW Jr, Arnold JM (eds) Squid as experimental animals. Plenum, New York, pp 153–159
Bezanilla F, Vergara J, Taylor RE (1982) Voltage clamping of excitable membranes. In: Ehrenstein G, Lecar H (eds) Methods of experimental physics, vol 20. Academic, New York, pp 445–511
Caputo C, Perozo E, Bezanilla F (1990) Chemical modification of squid axon K channels by p-hydroxymercuriphenylsulfonic acid (PHMPS). Biophys J 57:16a
Cole KS, Moore JW (1960) Potassium ion current in the squid giant axon: dynamic characteristic. Biophys J 1:1–14
Drews G, Rack M (1988) Modification of sodium and gating currents by amino group specific cross-linking and monofunctional reagents. Biophysical J 54:383–391
Frech GC, VanDongen AMJ, Schuster G, Brown AM, Joho RH (1989) A novel potassium channel with delayed rectifier properties isolated from rat brain by expression cloning. Nature 340:642–645
Gilly W, Armstrong CM (1982) Slowing of sodium channel opening kinetics in squid axon by extracellular zinc. J Gen Physiol 79:935–964
Gonzalez A, Bolaños P, Caputo C (1993) Effects of sulfhydryl inhibitors on non-linear membrane currents in frog skeletal muscle fibers. J Gen Physiol 101:425–451
Gray TM, Mathews BW (1984) Intrahelical hydrogen bonding of serine, threonine and cysteine residues withinα-helices and its relevance to membrane-bound proteins. J Mol Biol 175:75–81
Gregoret LM, Rader SD, Fletterick RJ, Cohen FE (1991) Hydrogen bonds involving sulfur atoms in proteins. Proteins Struct Funct Genet 9:99–107
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for the recording from cell and cell-free membrane patches. Pflügers Arch 391:85–100
Heginbotham L, Abramson T, MacKinnon R (1992) A functional connection between the pores of distantly related ion channels as revealed by mutant K+ channels. Science 258:1152–1155
Hunneus-Cox F, Fernandez HL, Smith BH (1966) Effects of redox and sulfhydryl reagents on the bioelectric properties of the giant axon of the squid. Biophys J 6:675–689
Keana JFW, Stämpfli R (1974) Effect of several “specific” chemical reagents on the Na+, K+ and leakage currents in voltage-clamped single nodes of Ranvier. Biochim Biophys Acta 373:18–33
Liman ER, Hess P, Weaver F, Koren G (1991) Voltage-sensing residues in the S4 region of a mammalian K+ channel. Nature 353:752–756
Llano I, Webb CK, Bezanilla F (1988) Potassium conductance of the squid giant axon: single channel studies. J Gen Physiol 92:179–196
Logothetis DE, Movahedi S, Satler C, Lindpaintner K, Nadal-Ginard B (1992) Incremental reductions of positive charge within the S4 region of a voltage-gated K+ channel result in corresponding decreases in gating charge. Neuron 8:531–540
Means GE, Feeney RE (1971) Chemical modification of proteins. Holden-Day, San Francisco, p 254
Meves H, Rubly N, Stämpfli R (1988) The action of arginine-specific reagents on ionic and gating currents in frog myelinated nerve. Biochim Biophys Acta 943:1–12
Papazian DM, Timpe LC, Jan YN, Jan LY (1991) Alteration of voltage-dependence of Shaker potassium channel by mutations in the S4 sequence. Nature 349:305–310
Perozo E, Jong DS, Bezanilla F (1991) Single channel studies of the phosphorylation of K+ channels in the squid giant axon. II. Non stationary conditions. J Gen Physiol 98:19–34
Rack M, Woll KH (1984) Effects of chemical modification of carboxyl groups on the voltage-clamped nerve fiber of the frog. J Membr Biol 82:41–48
Rack M, Hu S, Rubly N, Waschow C (1984) Effects of chemical modification of amino and sulfhydryl groups on the voltage-clamped frog node of Ranvier. Pflügers Arch 400:403–408
Rack M, Rubly N, Waschow C (1986) Effects of some chemical reagents on Na+ current inactivation in myelinated nerve fibers of the frog. Biophys J 50:557–564
Shrager P (1977) Slow sodium inactivation in nerve after exposure to sulfhydryl blocking reagents. J Gen Physiol 69:183–202
Spires S, Begenisich T (1989) Pharmacological and kinetic analysis of K channel gating currents. J Gen Physiol 93:263–283
Stimers JR, Bezanilla F, Taylor RE (1987) Sodium channel gating current. Origin of the raising phase. J Gen Physiol 89:521–540
Stühmer WF, Conti M, Suzuki H, Wang XD, Noda M, Yahagi N, Kubo H, Numa S (1989) Structural parts involved in activation and inactivation of the sodium channel. Nature 339:597–603
White MM, Bezanilla F (1985) Activation of squid axon K+ channels. Ionic and gating current studies. J Gen Physiol 85:539–554
Zagotta WN, Aldrich, RW (1990) Voltage-dependent gating of Shaker A-type potassium channels in Drosophila muscle. J Gen Physiol 95:29–60
Zuazaga C, Steinacker A, del Castillo J (1984) The role of sulfhydryl and disulfide groups of membrane proteins in electrical conduction and chemical transmission. Puerto Rico Health Sci J 3:125–139
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Caputo, C., Perozo, E. & Bezanilla, F. Chemical modification of squid axon K+ channel -SH groups with the organic mercurial compoundp-hydroxymercuriphenylsulfonic acid (PHMPS). Pflügers Arch. 428, 315–322 (1994). https://doi.org/10.1007/BF00724513
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00724513