Summary
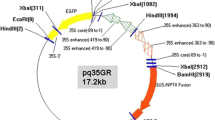
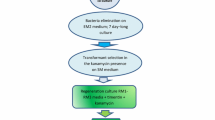
Daucus carota hypocotyl sections were transformed withAgrobacterium tumefaciens LBA4404 containing CaMV 35S promoter, β-glucuronidase coding sequence and the nopaline synthase (Nos) poly adenylation sequences in Bin 19. Sliced sterile seedling hypocotyl segments were preincubated for 2 days, co-cultivated withAgrobacterium for an additional 2 days, and then transferred to medium containing 100ug/ml of kanamycin and 400ug/ml carbenicillin. In 6 weeks kanamycin resistant calli were obtained in 5.8% of the explants from one variety. Calli were subcultured on solid medium, and in 4 weeks introduced into suspension culture. NPTII and Southern blot analysis confirmed that three selected lines were transformed with 1–3 copies of the GUSII construction. GUS activity in transformants was 5 to 250 fold over background.
Similar content being viewed by others
Abbreviations
- NPT II:
-
neomycin phosphotransferase II
- Nos:
-
nopaline synthase
- GUS:
-
β-glucuronidase
- CaMV:
-
cauliflower mosaic virus
- 2,4-D:
-
2,4 dichlorophenoxyacetic acid
- PMSF:
-
phenylmethylsulfonyl fluoride
References
Ammirato PV (1983) Embryogenesis. In: Evans DA, Sharp WR, Ammirato PV, Yamada T (eds) Handbook of Plant Cell Culture, Vol 1, Macmillan, New York, pp 87–123
Ammirato PV (1987) Organizational events during somatic embryogenesis. In: Green CE, Somers DA, Hackett WP, Biesboer DD (eds) Plant Tissue and Cell Culture, Alan Liss, New York, pp 57–81
Beachy R, Chen Z-L, Horsch R, Rogers S, Hoffmann N, Fraley R (1985) Accumulation and assembly of soybean ß-conglycinin in seeds of transformed petunia plants. EMBO J 4:3047–3053
Bolton GW, Nestor EW Gordon MP (1986) Plant phenolic compounds induce expression of theAgrobacterium tumefaciens loci needed for virulence. Science 232:983–985
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cardarelli M, Spano L, De Paolis A, Mauro ML, Vitali G, Costantino P (1985) Identification of the genetic locus responsible for non-polar root induction byAgrobacterium rhizogenes 1855. Plant Mol Biol 5:385–391
Charest PJ, Holbrook LA, Gabard J, Iyer VN, Miki BL (1988)Agrobacterium-mediated transformation of thin cell layer explants fromBrassica napus L. Theor Appl Genet 75:438–445
Choi JC, Liu L, Borkind C, Sung ZR (1987) Cloning of genes developmentally regulated during plant embryogenesis. Proc Natl Acad Sci 84:1906–1910
Dean CM, Favreau M, Tamaki S, Jones J, Dunsmuir P, Bedbrook J (1987) Expression of petuniarbcS gene fusions in transformed tobacco plants. In: Key JL, McIntosh L (eds) Plant Gene Systems and Their Biology, Alan Liss New York, pp 289–299
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gorman CM, Moffat LF, Howard BH (1982) Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol 2:1044–1051
Horsch RB, Fry JE, Hoffman NL, Wallroth M, Eichholtz DZ, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Jefferson RA, Kavanagh T, Bevan M (1987) The GUS gene fusion system. EMBO J 6:3901–3907.
Khandjian EW (1987) Optimized hybridization of DNA blotted and fixed to nitrocellulose and nylon membranes. Bio/Technology 5:165–167
Kozak M (1986) Point mutations define a sequence flanking the AUG initiator codon by eucaryotic ribosomes. Cell 44:283–292
Labarca C Paigen K (1980) A simple, rapid and sensitive DNA assay procedure. Anal Biochem 102:344–352
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, New York
Murashige T, Skoog F (1962) A revised medium for the rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Odell JT, Nagy F, Chua N-H (1987) Variability in 35S promoter expression between independent transformants. In: Key JL McIntosh L (eds) Plant Gene Systems and Their Biology, Alan Liss, New York pp 321–329
Pierce DA, Mettler IJ, Lachmansingh AR, Pomeroy LM, Weck EA, Mascarenhas D (1987) Effect of 35S Modifications on promoter activity. In: Key JL McIntosh L (eds) Plant Gene Systems and Their Biology, Alan Liss, New York pp 301–310
Reiss B, Sprengel R, Will H, Schaller H (1984) A new sensitive method for qualitative and quantitative assay of neomycin phosphotransferase in crude cell extracts. Gene 30:211–218
Ryder MH, Tate ME, Kerr A (1985) Virulence properties of strains ofAgrobacterium on the apical and basal surfaces of carrot root discs. Plant Physiol 77:215–221
Scott RJ Draper J (1987) Transformation of carrot tissues derived from proembryogenic suspension cells: A useful model system for gene expression in plants. Plant Mol Biol 8:265–274
Sengupta-Gopalan C, Reichart NA, Barker RF, Hall TC, Kemp JC (1985) Developmentally regulated expression of the bean β phaseolin gene in tobacco seed. Proc Natl Acad Sci 82:3320–3324
Steward FC, Mapes MO, Kent AE, Holsten RD (1964) Growth and development of cultured plant cells. Science 143:20–27
Sung ZR Okimoto R (1981) Embryonic proteins in somatic embryos of carrots. Proc Natl Acad Sci 78:3683–3687
Thomas JC, Nessler C, Katterman F (1989) Interruption of carrot somatic embryogenesis by 5-bromodeoxyuridine. Plant Physiol (In Press)
Ulian EC, RH Smith, JH Gould TD McKnight (1988) Transformation of plants via the shoot apex. In Vitro Cellular and Developmental Biology 24:951–954
Van Sluys MA, Tempe' J, Fedoroff N (1987) Studies on the introduction and mobility of the maizeActivator element inArabidopsis thaliana andDaucus carota. EMBO J 6:3881–3889
Wilde HD, Nelson WS, Booij H, De Vries S and Thomas TH (1988) Gene expression programs in embryogenic carrot cultures. Planta 176:205–211
Author information
Authors and Affiliations
Additional information
Communicated by J. M. Widholm
Rights and permissions
About this article
Cite this article
Thomas, J.C., Guiltinan, M.J., Bustos, S. et al. Carrot (Daucus carota) hypocotyl transformation usingAgrobacterium tumefaciens . Plant Cell Reports 8, 354–357 (1989). https://doi.org/10.1007/BF00716672
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00716672