Summary
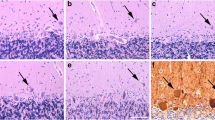
Ultrastructural and biochemical properties of caudate nucleus (CN) biopsies in two patients with advanced Parkinson's disease (PD) were compared with three CN specimens removed during surgery for intracranial tumors. An additional two specimens from neurologically intact patients (59 and 86 years old) were removed during autopsy (performed 3 and 4 h post mortem, respectively) for electron microscopic studies. Dopamine levels in PD were reduced to less than 15% of control values. Both PD patients showed frequent dystrophic neurites and transsynaptic degeneration of neurons and neuritic processes. These changes were not found in CN from the four control individuals. Only a few dystrophic neurites were noticed in one 67-year-old control patient. The development of neuroaxonal dystrophy in CN is consistent with a dying-back process, probably accompanying abnormalities of axonal transport in PD. Transsynaptic degeneration of neurons in CN very likely represents a morphological marker of disease severity. The occurrence of this change may account for the poor clinical response of patients with advanced PD to intracerebral implantation of dopaminergic tissues.
Similar content being viewed by others
References
Allen RD, Weiss DG, Hayden JH, Brown DT, Fujiwake H, Simpson M (1985) Gliding movement of and bidirectional transport along single native microtubules from squid axoplasm: evidence for an active role of microtubules in cytoplasmic transport. J Cell Biol 100:1736–1752
Backlund EO, Greenberg PO, Hamberger B, Knutsson E, Martensson A, Sedvall G, Seiger A, Olson L (1985) Transplantation of adrenal medullary tissue to striatum in parkinsonism. First clinical trial. J Neurosurg 62:169–173
Bancher C, Lassmann H, Budka H, Jellinger K, Grundke-Iqbal I, Iqbal K, Wiche G, Seitelbeger F, Wisniewski HM (1989) An antigenic profile of Lewy bodies: immunocytochemical indication for protein phosphorylation and ubiquitination. J Neuropathol Exp Neurol 48:81–93
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973) Brain dopamine and the syndromes of Parkinson and Huntington. J Neurol Sci 20:415–455
Blakemore WF, Cavanagh JB (1969) “Neuroaxonal dystrophy” occurring in an experimental “dying back” process in the rat. Brain 92:790–811
Bouyer JJ, Park DH, Joh TH, Pickel VM (1984) Chemical and structural analysis of the relation between cortical input and tyrosine hydroxylase containing terminals in rat neostriatum. Brain Res 302:267–275
Bugiani O, Perdelli F, Slavarani S, Leonardi A, Mancardi GL (1980) Loss of striatal neurons in Parkinson's disease: a cytometric study. Eur Neurol 19:339–344
Chou SM, Hartmann HA (1965) Electron Microscopy of focal neuroaxonal lesions produced by B,B1-iminodiproprionitrile (IDPN) in rats. Acta Neuropathol 4:590–603
Chung YW, Hasler RG (1984) Types of synapses in the pallidum and their differential degeneration following lesions of pallidal afferents in squirrel monkey (Saimiri sciurus). Adv Neurol 40:21–27
de Coster W, Roels H, Van der Eecken H (1971) Electron microscopical study of neuroaxonal dystrophy. Eur Neurol 5:65–83
Dickson DW, Wertkin A, Kress Y, Ksiezak-Reding H, Wen SH (1990) Ubiquitin immunoreactive structures in normal human brains. Distribution and developmental aspects. Lab Invest 63:87–99
Duffy P, Tennyson VM (1965) Phase and electronmicroscopic observations of Lewy bodies and melanin granules in the substantia nigra and locus ceruleus in Parkinson's disease. J Neuropathol Exp Neurol 24:398–414
Ehringer M, Hornykiewicz O (1950) Verteilung von Noradrenalin und Dopamin im Gehirn des Menschen und ihr Verhalten bei Erkrankungen des extrapyamidalen Systems. Klin Wochenschr 38:12–36
Forno SF (1986) The Lewy body in Parkinson's disease. Adv Neurol 45:35–43
Forno LS, Langston JW (1991) Unfavorable outcome of adrenal medullary transplant for Parkinson's disease. Acta Neuropathol 81:691–694
Forno LS, Norville RL (1979) Ultrastructure of neostriatum in Huntington's and Parkinson's disease. Adv Neurol 23:123–135
Forno LS, Sternberger LA, Sternberger NH, Strefling AM, Swanson K, Eng L (1986) Reaction of Lewy bodies with antibodies to phosphorylated and non-phosphorylated neurofilaments. Neurosci Lett 64:253–258
Freund TF, Powell JF, Smith AD (1984) Tyrosine hydroxylase-immunoreactive boutons in synaptic contacts with identified striato-nigral neurons, with particular reference to dendritic spines. Neuroscience 13:1189–1215
Fujisawa K, Shiraki H (1978) Study of axonal dystrophy. I. Pathology of the neuropil of the gracile and cuneate nuclei in ageing and old rats: a sterological study. Neuropathol Appl Neurobiol 4:1–20
Gajdusek DC (1985) Hypothesis: interference with axonal transport of neurofilament as a common pathogenetic mechanism in certain diseases of the central nervous system. N Engl J Med 312:714–719
Ghetti B, Wisniewski HM (1972) On degeneration of terminals in the cat striate cortex. Brain Res 44:630–635
Goldman JE (1987) Cytoskeletal abnormalities in Parkinson's disease. Adv Behav Biol 34:191–197
Goldstein M, Liebermann AN, Helmer E, Koslow M, Ransohoff J, Elsworth JD, Roth RH, Deutch AY (1988) Biochemical analysis of caudate nucleus biopsy samples from Parkinsonian patients. Ann Neurol 24:685–688
Gray EG, Hamlyn LH (1962) Electronmicroscopy of experimental degeneration in the avian optic tectum. J Anat 96:111–182
Griffin JW, Price DL, Engel WR, Drachman D (1977) The pathogenesis of reactive axonal swellings: role of axonal transport. J Neuropathol Exp Neurol 36:214–227
Griffin JW, Fahnestock KE, Price DL, Cork LC (1983) Cytoskeletal disorganization induced by local application of B,B1-iminodiproprionitrile and 2,5-hexanedione. Ann Neurol 14:55–61
Hassler R, Chung JW, Rinne U, Wagner A (1973) Selective degeneration of two out of nine types of synapses in cat caudate nucleus after cortical lesions. Exp Brain Res 31:67–80
Heidelberg-White ET, Floyd MB, Gilles H, Uzman GB (1968) Infantile neuroaxonal dystrophy. A disease characterized by altered terminal axons and synaptic endings. Neurology 18:891–906
Hornykiewicz O, Kish SJ (1986) Biochemical pathophysiology of Parkinson's disease. Adv Neurol 45:19–34
Hornykiewicz O, Pifl C, Schingnitz G, Kish SJ (1988) The cause of Parkinson's disease: MPTP, aging and the striatal dopamine loss. In: Noppi G, Hornykiewicz O, Fariello RG, et al (eds) Neurodegenerative disorders: the role played by endotoxins and xenobiotics. Raven Press, New York, pp 73–80
Hurtig H, Joyce J, Sladek J, Trojanowski JQ (1989) Postmortem analysis of adrenal-medulla-to-caudate autograft in a patient with Parkinson's disease. Ann Neurol 25:607–614
Javoy-Agid F, Ruberg M, Taquet H, Bokobza B, Agid Y, Gaspar P, Berger B, N'Guyen-Legros N, Alvarez C, Gray F, Escourolle R, Scatton B, Rouquier L (1984) Biochemical neuropathology of Parkinson's disease. Adv Neurol 40:189–198
Jellinger K (1973) Neuroaxonal dystrophy: its natural history and related disorders. Progr Neuropathol 2:129–180
Jellinger K (1987) Pathology of parkinsonism. In: Marsden CD, Fahn S (eds) Movement disorders, vol 2. Butterworth, London, pp 124–165
Jellinger K, Armstrong D, Zoghbi HY, Percy AK (1988) Neuropathology of Rett syndrome. Acta Neuropathol 76:142–158
Jones WH, Thomas DB (1962) Changes in the dendritic organization of neurons in the cerebral cortex following deafferentiation. J Anat 96:375–398
Kish SJ, Rajput A, Gilbert J, Rozdilsky B, Chang Li-Jan, Shannak K, Hornykiewicz O (1986) Elevated aminobutyric acid level in striatal but not extrastriatal brain regions in Parkinson's disease: correlation with striatal dopamine loss. Ann Neurol 20:26–31
Kish SJ, Shannak K, Hornykiewicz O (1988) Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. N Engl J Med 319:876–880
Lampert PW (1967) A comparitive electronmicroscopic study of reactive, degenerating, regenerating and dystrophic axons. J Neuropathol Exp Neurol 26:345–368
Lange HN, Bogerts B (1982) Postencephalitic and idiopathic Parkinsonism. Quantitative changes of tel-, di-, mesencephalum and basal ganglia. Neuroscience [Suppl] 17:127 (A)
Lewy FH (1913) Zur pathologischen Anatomie der Paralysis agitans. Dtsch Z Nervenheilke 50:50–55
MacDowell CM (1978) Fixation and processing. In: Trump BJ, Jones RT (eds) Diagnostic electron microscopy. Wiley Medical, Toronto, pp 113–140
Madrazo I, Druker-Colin R, Diaz V, Martinex-Mata J, Torres C, Becerril JJ (1987) Open microsurgical autograft of adrenal medulla to the right caudate nucleus in two patients with intractable Parkinson's disease. N Engl J Med 316:831–834
Mann DMA, Yates PO (1983) Pathological basis for neurotransmitter changes in Parkinson's disease. Neuropathol Appl Neurobiol 9:3–19
McNeill TH, Brown SA, Shoulson I, Lapham LW, Eskin TA, Rafols JA (1987) Regression of striatal dendrites in Parkinson's disease. In: Carpenter MB, Jayaraman A (eds) The basal ganglia, vol 2; structure and function. Plenum Press, New York, pp 475–482
Miller RH, Lasek RJ (1985) Cross bridges mediate anterograde and retrograde vesicle transport along microtubules in squid axoplasm. J Cell Biol 101:2181–2193
Nakamura Y (1982) Familial neuroaxonal dystrophy with principal lesions of nigro-pallido-subthalamic degeneration. Folia Psychiatr Neurol Jpn 36:151–162
Nyberg P, Nordbert A, Webster P, Windblad B (1983) Dopaminergic deficiency is more pronounced in putamen than in nucleus caudatus in Parkinson's disease. Neurochem Pathol 1:193–202
Papolla MA (1986) Lewy bodies of Parkinson's disease. Immune electron microscopic demonstration of neurofilament antigens in constituent filaments. Arch Pathol Lab Med 110:1160–1163
Pearson J (1983) Neurotransmitter immunocytochemistry in the study of human development, anatomy and pathology. Prog Neuropathol 5:41–97
Perlmutter JS (1988) New insights into the pathophysiology of Parkinson's disease: the challenge of positron emission tomography. Trend Neurosci 11:203–208
Peters A, Palay SL, Webster H, De F (1976) The fine structure of the nervous system. The neurons and supporting cells. W. B. Saunders, Toronto, pp 118–169
Pickel VM, Beckley SC, Joh TH, Reis DJ (1981) Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Res 225:373–385
Politis MJ, Pellegrino RG, Spence PS (1978) Ultrastructural studies of the dying back process. V. axonal neurofilament accumulation at the sites of 2,5-hexamenodione application: evidence for nerve fiber dysfunction in experimental hexocarbon neuropathy. J Neurocytol 9:505–516
Prineas J (1969) The pathogenesis of dying back polyneuropathies. I. An ultrastructural study of experimental triortho-cresyl phosphate intoxication in the cat. J Neuropathol Exp Neurol 28:571–597
Prineas J (1969) The pathogenesis of dying back polyneuropathies. II. Ultrastructural study of experimental acrylamide intoxication in the cat. J Neuropathol Exp Neurol 28:598–621
Sandbank U, Lerman P, Giefman M (1970) Infantile neuroaxonal dystrophy: cortical axonic and presynaptic changes. Acta Neuropathol (Berl) 16:342–352
Scatton B, Javoy-Agid F, Moutfort JC, Agid Y (1984) Neurochemistry of monoaminergic neurons in Parkinson's disease. In: Usdin E, Carlsson A, Dahlstorm A, Engel J (eds) Catecholamines: neuropharmacology and central nervous system — Therapeutic aspects. AR Liss, New York, pp 43–52
Schnapp BJ, Vale RD, Sheetz MP, Reese TS (1985) Single microtubules from squid axoplasm support bidirectional movement of organelle. Cell 40:4555–4562
Seegal FR, Brosch KO, Busch B (1986) High performance liquid chromatography of biogenic amines and metabolites in brain, CSF, urine and plasma. J Chromatogr 377:131–144
Seitelberger F (1971) Neuropathological conditions related to neuroaxonal dystrophy. Acta Neuropathol (Berl) [Suppl] V:17–29
Shermann D, Desnos C, Darchen F, Pollak P, Javoy-Agid F, Agid Y (1989) Stratal dopamine deficiency in Parkinson's disease: role of aging. Ann Neurol 26:551–557
Sotelo C, Palay SL (1971) Altered axons and axon terminals in the lateral vestibular nucleus of the rat. Possible example of axonal remodeling. Lab Invest 215:653–670
Spencer PS, Schumberg HH (1976) Central-peripheral axonopathy — The pathology of dying-back polyneuropathies. Prog Neuropathol 8:253–295
Stadlan EM, Duvoisin R, Yahr M (1965) The pathology of Parkinsonism. Excerpta Med Int Congr Ser 100:569–571
Sung SH (1965) Neuroaxonal dystrophy in aging. Excepta Med Int Congr Ser 100:478–480
Tennyson VM, Heikekila R, Mytilineou C, Cote L, Cohen G (1974) 5-Hydroxydopamine “tagged” neuronal boutons in rabbit striatum; inter-relationship between vesicles and axonal membranes. Brain Res 82:342–348
Wilson CJ, Groves PM (1980) Fine structure and synaptic connections of the common spinal neurons of neostriatum: as studied employing intracellular injection of horseradish proxidase. J Comp Neurol 194:599–615
Voorn P, Buijs RM (1987) Ultrastructural demonstration of dopamine in the central nervous system. In: Steinbuch HWM (ed) Monoaminergic neurons: light microscopy and ultrastructure. J. Wiley and Sons, New York, pp 241–264
Zalcman S, Shanks N, Anisman H (1991) Time-dependent variations of central norephinephrine and dopamine following antigen administration. Brain Res (in press)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lach, B., Grimes, D., Benoit, B. et al. Caudate nucleus pathology in Parkinson's disease: ultrastructural and biochemical findings in biopsy material. Acta Neuropathol 83, 352–360 (1992). https://doi.org/10.1007/BF00713525
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00713525