Summary
-
1.
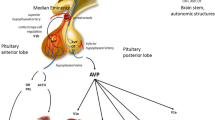
Regulatory interactions between the hypothalamoneurohypophyseal vasopressin (VP) axis and the endocrine systems of the anterior pituitary have been investigated in the rat by observing changes in VP mRNA expression following endocrine manipulations.
-
2.
An increase in the level, but not size, of VP mRNA was found in the supraoptic (SON) and paraventricular nuclei (PVN) of the hypothalamus and in the neurointermediate lobe (NIL) of the pituitary following hypothyroidism (induced by drinking 6-n-propyl-2-thiouracil; PTU) and adrenalectomy. Hypothyroidism induced by alternative procedures (surgical thyroidectomy or PTU injections) did not exert similar effects.
-
3.
Treatment with the dopamine agonist bromocriptine to reduce prolactin secretion raised levels of VP mRNA in the NIL only. Castration did not up-regulate VP mRNA levels.
-
4.
Since the observed effects on VP mRNA levels occur in the absence of changes in plasma osmolality, these results provide evidence of nonosmotic regulation of VP gene expression, an effect which is observed most clearly in the NIL pool of VP mRNA. Furthermore, the effects are distinct from changes in VP mRNA levels associated with raised plasma osmolality since the VP mRNA size was not increased.
Similar content being viewed by others
References
Aronsson, M., Fuxe, K., Dong, Y., Agnati, L. F., Okret, S., and Gustafsson, J.-A. (1988). Localization of glucocorticoid receptor mRNA in the male rat brain by in situ hybridization.Proc. Natl. Acad. Sci. USA 859331–9335.
Bergland, R. M., and Page, R. B. (1979). Pituitary-brain vascular relations: A new paradigm.Science 20418–24.
Boykin, J., DeTorrente, A., Erickson, A., Robertson, G., and Schrier, R. W. (1978). Role of plasma vasopressin in imparied water excretion of glucocorticoid deficiency.J. Clin. Invest. 62738–744.
Bradley, D. J., Young, W. S., III, and Weinberger, C. (1989). Differential expression of and thyroid hormone receptor genes in rat brain and pituitary.Proc. Natl. Acad. Sci. USA 867250–7254.
Carrazana, E. J., Pasieka, E. B., and Majzoub, J. A. (1988). The vasopressin mRNA poly(A) tail is unusually long and increases during stimulation of vasopressin gene expression in vivo.Mol. Cell. Biol 82267–2274.
Carter, D. A., and Lightman, S. L. (1985). Neuroendocrine control of vasopressin secretion. InThe Posterior Pituitary (P. H. Baylis and P. L. Padfield, eds.), Marcel Dekker, New York, pp. 53–118.
Carter, D. A., and Murphy, D. (1989). Independent regulation of neuropeptide mRNA level and poly(A) tail length.J. Biol. Chem. 2646601–6603.
Carter, D. A., and Murphy, D. (1991). Rapid changes in poly(A) tail length of vasopressin and oxytocin mRNAs form a common early component of neurohypophyseal peptide gene activation following physiological stimulation.Neuroendocrinology 531–6.
Davis, L. G., Arentzen, R., Reid, J. M., Manning, R. W., Wolfson, B., Lawrence, K. L., and Baldino, F., Jr. (1986). Glucocorticoid sensitivity of vasopressin mRNA levels in the paraventricular nucleus of the ratProc. Natl. Acad. Sci. USA 831145–1149.
Gillies, G. E., Linton, E. A., and Lowry, P. J. (1982). Corticotrophin releasing activity of the new CRF is potentiated several times by vasopressin.Nature 299355–357.
Holmes, M. C., Antoni, F. A., Aguilera, G., and Catt, K. J. (1986). Magnocellular axons in passage through the median eminence release vasopressin.Nature 319326–329.
Kiss, J. Z., Mezey, E., and Skirboll, L. (1984). Corticotropin-releasing factor immunoreactive neurons of the paraventricular nucleus become vasopressin positive after adrenalectomy.Proc. Natl. Acad. Sci. USA 811854–1858.
Kiss, J. Z., van Eekelen, J. A. M., Reul, J. M. H. M., Westphal, H. M., and De Kloet, E. R. (1988). Glucocorticoid receptor in magnocellular neurosecretory neurons.Endocrinology 122444–449.
Labrie, F., Ferland, L., Denizeau, F., and Beaulieu, M. (1980). Sex steroids interact with dopamine at the hypothalamic and pituitary levels to modulate prolactin secretion.J. Steroid Biochem. 12323–330.
Ladenson, P. W., Kieffer, J. D., Farwell, A. P., and Ridgway, E. C. (1986). Modulation of mycocardial L-triiodothyronine receptors in normal, hypothyroid and hyperthyroid rats.Metabolism 355–12.
Levy, A., Lightman, S. L., Carter, D. A., and Murphy, D. (1990). The origin and regulation of posterior pituitary vasopressin in ribonucleic acid in osmotically stimulated rats.J. Neuroendocrinol. 2329–324.
Linas, S. L., Berl, T., Robertson, G. L., Aisenbrey, G. A., Schrier, R. W., and Anderson, R. J. (1980). Role of vasopressin in the impaired water excretion of glucocorticoid deficiency.Kidney Int. 1858–67.
Lumpkin, M. D., Samson, W. K., and McCann, S. M. (1987). Arginine vasopressin as a thyrotropin-releasing hormone.Science 2351070–1073.
Mohr, E., Fehr, S., and Richter, D. (1991). Axonal transport of neuropeptide encoding mRNAs within the hypothalamo-neurohypophyseal tract of rats.EMBO J. 102419–2424.
Murphy, D., and Carter, D. A. (1990). Vasopressin gene expression in the rodent hypothalamus: Transcriptional and post-transcriptional responses to physiological stimulation.Mol. Endocrinol. 41051–1059.
Murphy, D., Levy, A., Lightman, S., and Carter, D. A. (1989). Vasopressin RNA in the neural lobe of the pituitary: Dramatic accumulation in response to salt loading.Proc. Natl. Acad. Sci. USA 869002–9005.
Murphy, D., Pardy, K., Seah, V., and Carter, D. A. (1992). Post-transcriptional regulation of rat growth hormone gene expression: Increased message stability and nuclear polyadenylation accompany thyroid hormone depletion.Mol. Cell. Biol. 122624–2632.
Nagy, G., Mulchahey, J. J., Smyth, D. G., and Neill, J. D. (1988). The glycopeptide moiety of vasopressin-neurophysin precursor is neurohypophysial prolactin releasing factor.Biochem. Biophys. Res. Comm. 151524–529.
Ouafik, L., May, V., Saffen, D. W., and Eipper, B. A. (1990). Thyroid hormone regulation of peptidylglycineα-amidating monoxygenase expression in anterior pituitary gland.Mol. Endocrinol. 41497–1505.
Palkovits, M. (1973). Isolated removal of hypothalamic or other brain nuclei of the rat.Brain Res. 59449–450.
Richter, D. (1988). Molecular events in expression of vasopressin and oxytocin and their cognate receptors.Am. J. Physiol. 255F207-F219.
Robinson, I. C. A. F. (1986). The magnocellular and parvocellular OT and AVP systems. InNeuroendocrinology (S. L. Lightman and B. J. Everitt, eds.) Blackwell Scientific, Oxford, pp. 154–176.
Schilling, K., Schmale, H., Oeding, P., and Pilgrim, C. (1991). Regulation of vasopressin expression in cultured diencephalic neurons by glucocorticoids.Neuroendocrinology 53528–535.
Schrier, R. W., Berl, T., and Anderson, R. J. (1979). Osmotic and non-osmotic control of vasopressin release.Am. J. Physiol. 236F321-F332.
Seif, S. M., Robinson, A. G., Zimmerman, E. A., and Wilkins, J. (1978). Plasma neurophysin and vasopressin in the rat: Response to adrenalectomy and steroid replacement.Endocrinology 1031009–1015.
Seif, S. M., Robinson, A. G., Zenser, T. W., Davis, B. B., Huellmantel, A. B., and Haluszczak, C. (1979). Neurohypophyseal peptides in hypothyroid rats: Plasma levels and kidney response.Metabolism 28137–143.
Skowsky, W. R., and Gisher, D. A. (1977). Arginine vasopressin secretion in thyroidectomized sheep.Endocrinology 1001022–1026.
Suemaru, S., Hashimoto, K., Ogasa, T., Takao, T., Ota, Z., Hirakawa, M., and Kawata, M. (1990). Effects of hyperosmotic stimulation and adrenalectomy on vasopressin mRNA levels in the paraventricular and supraoptic nuclei of the hypothalamus: In situ hybridization histochemical analysis using a synthetic oligonucleotide probe.Acta Med. Okayama 5233–241.
Zimmerman, E. A., and Silverman, A. J. (1983). Vasopressin and adrenal cortical interactions. InThe Neurohypophysis: Structure, Function and Control, Progress in Brain Research, Vol. 60 (B. A. Cross and G. Leng, Eds.), Elsevier, Amsterdam, pp. 493–504.
Zingg, H. H., Lefebvre, D. L., and Almazan, G. (1988). Regulation of poly(A) tail size of vasopressin mRNA.J. Biol. Chem. 26311041–11043.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Carter, D.A., Pardy, K. & Murphy, D. Regulation of vasopressin gene expression: Changes in the level, but not the size, of vasopressin mRNA following endocrine manipulations. Cell Mol Neurobiol 13, 87–95 (1993). https://doi.org/10.1007/BF00712991
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00712991