Summary
-
1.
Investigations have demonstrated that the gene encoding thymosinβ 10 (a 43-amino acid member of a family of related proteins originally described in the rat immune system) is a target for morphogenic retinoids in both human and rat neuroblastoma cells.
-
2.
Structure-activity studies revealed that the stimulatory actions of retinoids upon the thymosinβ 10 gene reflect the differing affinities of retinoid analogues for a retinoic acid receptor.
-
3.
To examine further the possibility that the trophic actions of retinoic acid upon expression of the thymosinβ 10 gene involved retinoid receptors, neuroblastoma cells were transiently transfected with an expression vector encoding the nuclear retinoic acid receptor (α) protein.
-
4.
Northern blot and slot-blot analyses revealed that neuronal cells overexpressing RARα-mRNA exhibited an enhanced sensitivity to exogenous and endogeneous retinoic acid in terms of thymosinβ 10 mRNA. Although the RAR-α gene was expressed (at low levels) a priori in these neuroblastoma cells, retinoic acid (2 × 10−7 M for 3 days) slightly stimulated RAR-α-mRNA accumulation.
-
5.
Collectively, these findings indicate the the retinoic acid receptor (α) is regulated by retinoid acid and that the developmentally regulated, retinoidresponsive thymosinβ 10 gene is a target for this nuclear transcription factor in cells derived from the neural crest.
Similar content being viewed by others
References
Astrom, A., Pettersson, U., Krust, A., Chambon, P., and Voorhees, J. J. (1991). Retinoic acid and synthetic analogs differentially activate retinoic acid receptor dependent transcription.Biochem. Biophys. Res. Comm. 173339–345.
Brand, N. J., Petrovich, M., Krust, A., Chambon, P., De Thé, H., Marchio, A., Tiollais, P., and Dejean, A. (1988). Identification of a second human retinoic acid receptor.Nature (Lond.)322850–853.
Cadi, R., Pautou, M.-P., and Dhouailly, D. (1984). Structure-activity relationships of retinoids in the morphogenesis of cutaneous appendages in the chick embryo.J. Invest. Dermatol. 83105–109.
Chomczynski, P., and Sacchi, N. (1987). Single-step method of RNA isolation by acid guanidiniumthiocyanate-phenol-chloroform extraction.Anal. Biochem. 162156–159.
Condon, M. R., Lyse, T., Seebode, J. J., and Hall, A. K. (1990). Preliminary characterization of the human thymosinβ 10 gene and its expression in the developing human brain.Soc. Neurosci. Abstr. 16(2):663.
Durston, A. J., Timmermans, J. P. M., Hage, W. J., Hendriks, H. F. J., de Vries, N. J., Heideveld, M., and Nieuwkoop, P. D. (1989). Retinoic acid causes an anterioposterior transformation in the developing central nervous system.Nature 340140–144.
Feinburg, A. P., and Vogelstein, B. (1984). A technique for radiolabelling DNA restriction enodnuclease fragments to high specific activity.Anal. Biochem. 256402–405.
Giguere, V., Ong, E. S., Sequi, P., and Evans, R. M. (1987). Identification of a receptor for the morphogen retinoic acid.Nature (Lond.) 330624–629.
Green, S., Issemann, I., and Sheer, E. (1988). A versatilein vivo andin vitro expression vector for protein engineering.Nucl. Acid. Res. 16(1):396.
Grippo, J. F., and Gudas, L. J. (1987). The effect of dibutyryl cyclic AMP and butyrate on F9 teratocarcinoma cellular retinoic acid-binding protein activity.J. Biol. Chem. 2624492–4500.
Hall, A. K. (1991a). Development regulation of thymosin beta-10 mRNA in the human brain.Mol. Brain Res. 9175–177.
Hall, A. K. (1991b). Retinoic acid and serum modulation of thymosin beta-10 gene expression in rat neuroblastoma cells.J. Mol. Neurosci. 2229–237.
Hall, A. K. (1991c). Differential expression of thymosin genes in human tumors and in the developing human kidney.Int. J. Cancer 48672–677.
Hall, A. K., Morgan, J. I., and Seebode, J. J. (1989). Differentiation-associated modulation of thymosin beta-10 expression in neuroblastoma cells by retinoic acid. InAdvances in Gene Technology; Molecular Neurobiology and Neuropharmacology, Vol. 1 Cambridge University Press, Cambridge, p. 70.
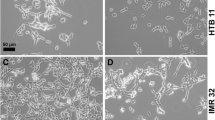
Hall, A. K., Hempstead, J., and Morgan, J. I. (1990). Retinoic beta-10 levels in developing human brain and its regulation by retinoic acid in the HTB-10 neuroblastoma.Mol. Brain Res. 8129–135.
Hall, A. K., Hempstead, J., and Morgan, J. I. (1991a). Retinoic acid regulates thymosin beta-10 levels in rat neuroblastoma cells.J. Neurochem. 56462–468.
Hall, A. K., Fernandes, M., and Seebode, J. J. (1991b). Thymosin beta-10: An oncofetal, retinoid-responsive gene expressed in human embryonic tissues and tumors.Miami Short Rep. 177.
Hall, A. K., Aten, R., and Behrman, H. R. (1991c). Thymosin gene expression is modulated by pregnant mare's serum gonadotropin, human chorionic gonadotropin and prostaglandin F in the immature rat ovary.Endocrinology 128951–957.
Horecker, B. L., and Morgan, J. I. (1984). Ubiquitous distribution of thymosin beta-4 and related peptides in vertebrate cells and tissues.Lymphokines 915–35.
Keeble, S., and Maden, M. (1989). The relationship among retinoid structure, affinity for retinoic acid-binding protein, and ability to respecify pattern in the regenerating axolotl limb.Dev. Biol. 13226–34.
Leonard, D. G. B., Ziff, E. B., and Greene, L. A. (1987). Identification and characterization of mRNAs regulated by nerve growth factor in PC12 cells.Mol. Cell. Biol. 73156–3167.
Leroy, P., Kurst, A., Zelent, A., Mendelsohn, C., Garnier, J.-M., Kastner, P., Dierich, A., and Chambon, P. (1981). Multiple isoforms of the mouse retinoic acid receptor-α induction by retinoic acid.EMBO J. 1059–69.
Lin, S.-C., and Morrison-Bogorad, M. (1990). Developmental expression of mRNAs encoding thymosins beta-4 and beta-10 in rat brain and other tissues.J. Mol. Neurosci. 235–44.
Lugo, D. L., Chen, S.-C., Hall, A. K., Hempstead, J., Ziai, R., and Morgan, J. I. (1991). Developmental regulation ofβ-thymosins in the rat central nervous system.J. Neurochem. 56456–461.
Mangelsdorf, D. J., Ong, E. S., Dyck, J. A., and Evans, R. M. (1990). Nuclear receptor identifies a novel retinoic acid response pathway.Nature 345224–229.
Momoi, T., Kitamoto, T., Seno, H., and Momoi, M. (1988). The distribution of cellular retinoic acid binding protein (CRABP) in the central nervous system of the chick embryo during development.Proc. Jpn. Acad. 64294–297.
Oro, A. E., McKeown, M., and Evans, R. M. (1990). Relationship between the product of theDrosophila ultraspiracle locus and the vertebrate retinoid X receptor.Nature 347298–301.
Petrovich, M., Brand, N. J., Kurst, A., and Chambon, P. A. (1987). A human retinoic acid receptor which belongs to a family of nuclear receptors.Nature 330444–450.
Picard, D., Khursheed, B., Garabedian, M. J., Fortin, M. G., Lindquist, S., and Yamamoto, K. R. (1990). Reduced levels of hsp90 compromises steroid receptor actionin vivo.Nature 348166–168.
Safer, D., Elzinga, M., and Nachmias, V. T. (1991). Thymosinβ4 and Fx, an actin-sequestering peptide, are indistinguishable.J. Biol. Chem. 2664029–4032.
Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989). InMolecular Cloning, a Laboratory Manual, Vol. 1, Cold Springer Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Thé, H., De., Vivanco-Ruize, M. D. M., Tiollais, P., Stunnenberg, H., and Dejean, A. (1990). Identification of a retinoic acid responsive element in the retinoic acid receptor-β gene.Nature 343177–180.
Thomas, P. (1980). Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose.Proc. Natl. Acad. Sci. 775201–5205.
Zelent, A., Krust, A., Petrovich, M., Kastner, P., and Chambon, P. A. (1989). Cloning of murineα andβ retinoic acid receptors and a novel receptorγ predominantly expressed in skin.Nature 339714–717.
Zelent, A., Mendelsohn, C., Kastner, P., Krust, A., Garnier, J.-M., Ruffenach, F., Leroy, P., and Chambon, P. (1991). Differentially expressed isoforms of the mouse retinoic acid receptorβ are generated by usage of two promoters and alternative splicing.EMBO J. 1071–81.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hall, A.K. Retinoids and a retinoic acid receptor differentially modulate thymosinβ 10 gene expression in transfected neuroblastoma cells. Cell Mol Neurobiol 12, 45–58 (1992). https://doi.org/10.1007/BF00711638
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00711638