Summary
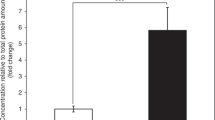
Phytanic acid α-oxidation was studied in cultures of skin fibroblasts and myoblasts from patients with various defects of the respiratory chain in order to obtain information on the subcellular site and the mechanism of this pathway. In fibroblasts from patients with complex IV (cytochromec oxidase) deficiency or glutaricaciduria type II, phytanic acid α-oxidation was reduced to 14% of normal, whereas in myoblasts from patients with complex I (NADH-Q reductase) deficiency, it was normal. Apparently, at least one step of phytanic acid α-oxidation occurs in mitochondria and in this process electrons are transferred to the respiratory chain via the electron-transfer flavoprotein (ETF).
Similar content being viewed by others
References
Beard ME, Sapirstein V, Kolodny EH, Holtzman E (1985) Peroxisomes in fibroblasts from skin of Refsum's disease patients.J Histochem Cytochem 33: 480–484.
Fingerhut R, Schmitz W, Conzelmann E (1993) Accumulation of phytanic acid α-oxidation intermediates in Zellweger fibroblasts.J Inher Metab Dis 16: 591–594.
Goldfischer S, Moore CL, Johnson AB et al (1973) Peroxisomal and mitochondrial defects in the cerebo-hepato-renal syndrome.Science 182: 62–64.
Huang S, van Veldhoven PP, Vanhoutte F, Parmentier S, Eyssen HJ, Mannaerts GP (1992) α-Oxidation of 3-methyl-substituted fatty acids in rat liver.Arch Biochem Biophys 296: 214–223.
Jung ME, Ornstein PL (1977) A new method for the efficient conversion of alcohols into iodides via treatment with trimethylsilyl iodide.Tetrahedron Lett 31: 2659–2662.
Klenk E, Kahlke W (1963) Über das Vorkommen der 3,7,11,15-Tetramethylhexadecansäure (Phytansäure) in den Cholesterinestern und anderen Lipoidfraktionen der Organe bei einem Krankheitsfall unbekannter Genese (Verdacht auf Heredopathia atactica polyneuritiformis, Refsum's Syndrom).Hoppe-Seyler's Z Physiol Chem 333: 133–139.
Lazarow PB, Moser HW (1989) Disorders of peroxisome biogenesis. In Scriver CR, Beaudet AL, Sly WS, Valle D, eds.The Metabolic Basis of Inherited Disease, 6th edn. New York: McGraw-Hill, 1479–1509.
Poll-The BT, Skjeldal OH, Stokke O, Poulos A, Demaugre F, Saudubray J-M (1989) Phytanic acid alpha-oxidation and complementation analysis of classical Refsum and peroxisomal disorders.Hum Genet 81: 175–181.
Poulos A (1981) Diagnosis of Refsum's disease using [1-14C]phytanic acid as substrate.Clin Genet 20: 247–253.
Poulos A, Barone E, Johnson DW (1980) Partial synthesis of [1-14C]phytanic acid.Lipids 15: 19–21.
Poulos A, Sharp P, Whiting M (1984) Infantile Refsum's disease (phytanic acid storage disease): a variant of Zellweger's syndrome?Clin Genet 26: 579–586.
Schmitz W, Fingerhut R, Conzelmann E (1994) Purification and properties of an α-methylacyl-CoA racemase from rat liver.Eur J Biochem 222:313–323.
Singh I, Lazo O, Kalipada P, Singh AK (1992) Phytanic acid α-oxidation in human cultured skin fibroblasts.Biochim Biophys Acta 1180: 221–224.
Skjeldal OH, Stokke O (1987) The subcellular localization of phytanic acid oxidase in rat liver.Biochim Biophys Acta 921: 38–42.
Steinberg D (1989) Refsum disease. In Scriver CR, Beaudet AL, Sly WS, Valle D, eds.The Metabolic Basis of Inherited Disease, 6th edn. New York: McGraw-Hill, 1533–1550.
Ten Brink HJ, Schor DSM, Kok RM, Poll-The BT, Wanders RJA, Jakobs C (1992) Phytanic acid α-oxidation: accumulation of 2-hydroxyphytanic acid and absence of 2-oxophytanic acid in plasma from patients with peroxisomal disorders.J Lipid Res 33: 1449–1457.
Trijbels JMF, Berden JA, Monnens LAH et al (1983) Biochemical studies in the liver and muscle of patients with Zellweger syndrome.Pediatr Res 17: 514–517.
Wanders RJA, van Roermund CWT (1993) Studies on phytanic acid α-oxidation in rat liver and cultured human skin fibroblasts.Biochim Biophys Acta 1167: 345–350.
Watkins PA, Mihalik SJ, Skjeldal OH (1990) Mitochondrial oxidation of phytanic acid in human and monkey liver: implication that Refsum's disease is not a peroxisomal disorder.Biochem Biophys Res Commun 167: 580–586.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fingerhut, R., Schmitz, W., Garavaglia, B. et al. Impaired degradation of phytanic acid in cells from patients with mitochondriopathies: Evidence for the involvement of ETF and the respiratory chain in phytanic acid α-oxidation. J Inherit Metab Dis 17, 527–532 (1994). https://doi.org/10.1007/BF00711585
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00711585