Abstract
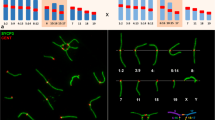
About 50 copies of a long-range repeat DNA family with a repeat size of roughly 100 kb and with sequence homology to mRNAs are clustered in the G-light band D of chromosome 1 of the house mouse,Mus musculus. We studied amplified versions of the cluster which are found in many wild populations ofM. musculus. They are cytogenetically conspicuous as one or two C-band positive homogeneously staining regions (single- and double band HSRs) which increase the mitotic length of chromosome 1. The double band HSR was phylogenetically derived from a single band HSR by a paracentric inversion. In homozygous condition, such HSRs contribute, albeit not as much as expected from their mitotic length, to the synaptonemal complex (SC) length of chromosome 1. In HSR heterozygous animals an elongation of the SCs was not noticeable. In single band HSR heterozygous males, synapsis proceeds regularly and continuously from the distal telomere towards the centromeric end without forming buckles. Thus, the single band HSR has no adverse effect on pairing. The same straight pairing behaviour was found in the majority of double band HSR heterozygous spermatocytes. This shows that extensive nonhomologous pairing can take place in the earliest phase of synapsis. Synapsis was discontinuous, leaving the central part of the bivalent 1 asynapsed, in only 14.3% of double band HSR heterozygous cells. In such cells the chromosome 1 SC is completed at a later stage of meiosis. The delay is presumably an effect of the inversion that includes one HSR band and the segment between the two HSR bands.
Similar content being viewed by others
References
Adolph S, Klett C, Weith A (1990) Nonradioactivein situ nick translation combined with counterstaining: Characterization of C-band and silver positive regions in mouse testicular cells.Chromosoma 99: 251–259.
Albini SM, Jones GH, Wallace BMN (1984) A method for preparing two-dimensional surface-spreads of synaptonemal complexes from plant meiocytes for light and electron microscopy.Exp Cell Res 152: 280–285.
Ashley T (1988) G-band position effects on meiotic synapsis and crossing over.Genetics 118: 307–317.
Borodin PM, Gorlov IP, Ladygina TY (1990) Synapsis in single and double heterozygotes for partially overlapping inversions in chromosome 1 of the house mouse.Chromosoma 99: 365–370.
de Boer (1986) Chromosomal causes for fertility reduction in mammals. In: de Serres FJ, ed.Chemical Mutagens 10. Plenum Publishing Corporation, pp 427–467.
de Boer P, de Jong JH (1986) Chromosome pairing and fertility in mice. In: Gillies CB, ed.Fertility and Chromosome Pairing: Recent Studies in Plant and Animals. Boca Raton: CRC Press, pp 37–76.
Dietrich AJJ, Mulder RJP (1981) A light microscopic study of the development and behaviour of the synaptonemal complex in spermatocytes of the mouse.Chromosoma 83: 409–418.
Eckert WA, Plass C, Weith Aet al. (1991) Transcripts from amplified sequences of an inherited homogeneously staining region in chromosome 1 of the house mouse (Mus musculus).Mol Cell Biol 6: 478–491.
Evans EP, Breckon G, Ford CE (1964) An air-drying method for meiotic preparations from mammalian testes.Cytogenetics 3: 289–294.
Fletcher JM (1979) Light microscope analysis of meiotic prophase chromosomes by silver staining.Chromosoma 72: 241–248.
Gillies CB (1985) An electron microscopic study of synaptonemal complex formation at zygotene in rye.Chromosoma 92: 165–175.
Goetz P, Chandley AC, Speed RM (1984) Morphological and temporal sequence of meiotic prophase development at puberty in the male mouse.J Cell Sci 65: 249–263.
Grao P, Coll MD, Ponsa Met al. (1989) Trivalent behaviour during prophase I in male mice heterozygous for three Robertsonian translocations: an electron-microscopic study.Cytogenet Cell Genet 52: 105–110.
Hale DW, Greenbaum IF (1988) Synapsis of a chromosomal pair heterozygous for a pericentric inversion and the presence of a heterochromatic short arm.Cytogenet Cell Genet 48: 55–57.
Jones GH, Croft JA (1986) Surface spreading of synaptonemal complexes in locusts—II. Zygotene pairing behaviour.Chromosoma 93: 489–495.
King M (1987) Chromosomal rearrangements, speciation and the theoretical approach.Heredity 59, 1–6.
Moriwaki K, Miyashita N, Suzuki Het al. (1986) Genetic features of major geographic isolates of Mus musculus.Curr Top Microbiol Immunol 127: 55–61.
Moses MJ (1977) Synaptonemal complex karyotyping in spermatocytes of the Chinese hamster (Cricetulus griseus). I. Morphology of the autosomal complement in spread preparations.Chromosoma 60: 127–137.
Moses MJ, Poorman PA, Roderick THet al. (1982) Synaptonemal complex analysis of mouse chromosome rearrangements. IV. Synapsis and synaptic adjustment in two paracentric inversions.Chromosoma 84: 457–474.
Purmann L, Plass C, Grüneberg Met al. (1992) A long-range repeat cluster in chromosome 1 of the house mouse,Mus musculus, and its relation to a germline homogeneously staining region.Genomics 12: 80–88.
Stack SM (1984) Heterochromatin, the synaptonemal complex and crossing over.J Cell Sci 71: 159–176.
Sudman PD, Greenbaum IF, Hale DWet al. (1989) Synaptic adjustment inPeromyscus beatae (Rodentia: Cricetidae) heterozygous for interstitial heterochromatin.Cytogenet Cell Genet 50: 1–5.
Sumner AT (1972) A simple technique for demonstrating centromeric heterochromatin.Exp Cell Res 75: 304–306.
Traut W, Winking H, Adolph S (1984) An extra segment in chromosome 1 of wildMus musculus: A C-band positive homogeneously staining region.Cytogenet Cell Genet 38: 290–297.
Traut W, Seldin MF, Winking H (1992) Genetic assignment of a long-range repeat cluster to band D of Chromosome 1 inMus musculus andM. spretus.Cytogenet Cell Genet 60: 128–130.
Traut W, Winking H, Plass Cet al. (1993) An inherited homogeneously staining region derived from a long-range repeat family in the house mouse. In: Obe G, Natarajan AT, eds.Chromosomal Alterations, Origin and Significance. Springer Verlag, in press.
Winking H, Weith A, Boldyreff Bet al. (1991a) Polymorphic HSRs in chromosome 1 of the two semispeciesMus musculus musculus andM. m. domesticus have a common origin in an ancestral population.Chromosoma 100: 147–151.
Winking H, Bostelmann H, Fredga K (1991b) Incidence of double band HSRs in chromosome 1 of the house mouse,Mus musculus musculus, from Öland (Sweden): a population study.Hereditas 114: 11–116.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Winking, H., Reuter, C. & Traut, W. Meiotic synapsis of homogeneously staining regions (HSRs) in chromosome 1 ofMus musculus . Chromosome Res 1, 37–44 (1993). https://doi.org/10.1007/BF00710605
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00710605