Summary
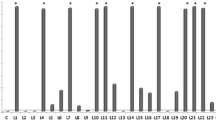
Volatile metabolites from a number of rhizosphere pseudomonads prevented lettuce root growth in a seedling bioassay. One of these metabolites was identified as cyanide. Direct contact between rhizobacteria and plant roots produced, with one exception, similar responses. However, not all cyanogenic isolates were plant-growth-inhibitory rhizobacteria. When grown in liquid culture, cyanogenic strains produced an average of 37 nmol HCN ml−1 over a 36-h period and inhibition of root growth occurred at concentrations as low as 20 nmol ml−1. Cyanogenic strains introduced into sand or soil also produced HCN. Two cyanogenic strains ofPseudomonas fluorescens, one (5241) a plant-growth inhibitory rhizobacterium and the other (S97) a plant-growth-promotory rhizobacterium, were used to treat bean and lettuce seedlings prior to planting in soil. Lettuce dry weight was reduced by 49.2% (day 28) and 37.4% (day 49) when inoculated with S241 whereas S97 increased growth initially (+64.5% at day 28, no difference from control at day 49). Equivalent figures for inoculated bean plants were: −52.9% and −65.1% (5241); +40.7% and +23.3% (S97). A more detailed experiment using only bean plants confirmed these contrasting affects. Inhibition by S241 was related to consistently higher levels of rhizosphere cyanide in comparison with S97-treated plants and control soils. S241 also survived in the rhizosphere at higher densities and for a longer period of time than S97. The possible contribution of rhizobacterial cyanogenesis to plant growth inhibition is discussed.
Similar content being viewed by others
References
Alström S (1987) Factors associated with detrimental effects of rhizobacteria on plant growth. Plant and Soil 102:3–9
Bakker AW, Schippers B (1987) Microbial cyanide production in the rhizosphere in relation to potato yield reduction andPseudomonas spp-mediated plant growth-stimulation. Soil Biol Biochem 19:451–458
Campbell NJ, Conn K, Sorlic L, Cook DF (1986) Inhibition of growth in canola seedlings caused by an opportunisticPseudomonas sp. under laboratory and field conditions. Can Microbiol 32:201–207
Castric AP (1975) Hydrogen cyanide, a secondary metabolite ofPseudomonas aeruginosa. Can J Microbiol 21:612–619
Dartnall AM, Burns RG (1987) A sensitive method for measuring cyanide and cyanogenic glucosides in sand culture and soil. Biol Fertil Soils 5:141–147
Fredrickson JK, Elliot LF (1985a) Colonization of winter wheat seedling growth by toxin-producing rhizobacteria. Plant and Soil 83:399–409
Fredrickson JK, Elliot LF (1985b) Colonization of winter wheat roots by inhibitory rhizobacteria. J Soil Sci Soc Am 49:1172–1176
Gardner MJ, Chandler LJ, Feldman WA (1984) Growth promotion and inhibition by antibiotic-producing fluorescent pseudomonads on citrus roots. Plant and Soil 77:103–113
Gerhardson B, Alstrom S, Ramert B (1985) Plant reactions to inoculation of roots with fungi and bacteria. Phytopathol Z 114:108–117
Hutchinson SA (1973) Biological activities of fungal metabolities. Annu Rev Phytopath 11:233–246
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44:301–307
Kloepper JW (1979) The role of rhizobacteria in increasing plant growth and yield. Ph. D. Thesis, University of Berkeley, Microfilms Int No 8014761
Knowles CJ (1976) Microorganisms and cyanide. Bacteriol Rev 40:652–680
Lambert JL, Ramasamy J, Pauksteilis JV (1975) Stable reagents for the colorimetric determination of cyanide by modified Konig reactions. Anal Chem 47:916–918
Leisinger T, Margraff R (1979) Secondary metabolities of the fluorescent pseudomonads. Microbiol Rev 43:422–442
Lorck H (1948) Production of hydrocyanic acid by bacteria. Physiol Plant 1:142–146
Miller JM, Conn EE (1980) Metabolism of hydrogen cyanide by higher plants. Plant Physiol 65:1199–1202
Schaad NM (1980) Laboratory guide for identication of plant pathogenic bacteria. Am Phytopathol Soc, Minnesota
Schippers B, Bakker AW, Bakker PAHM, Weisbeek PJ, Lugtenberg B (1986) Plant growth-inhibiting and stimulating rhizosphere microorganisms. In: Jensen V, Kjøller A, Sørensen LH (eds) Proc FEMS Symp Microbial Communities in Soil, Copenhagen 1985, Elsevier, London, pp 35–48
Traquair AJ, McKeen WE (1986) Fine structure of root tip cells of winter wheat exposed to toxic culture filtrates ofCoprinus psychromorbidus andMarasmius oreades. Can J Plant Pathol 8:59–64
Wissing F (1968) Growth curves and pH-optima for cyanide producing bacteria. Physiol Plant 21:589–593
Ward EWB, Lebean JB, Cormack MW (1961) Grouping of isolates of low-temperature Basidiomycetes on the basis of cultural behaviour and pathogenicity. Can J Bot 39:297–306
Wright SJL, Thompson RJ (1985)Bacillus volatiles antagonize cyanobacteria. FEMS Microbiol Lett 30:263–267
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Alström, S., Burns, R.G. Cyanide production by rhizobacteria as a possible mechanism of plant growth inhibition. Biol Fert Soils 7, 232–238 (1989). https://doi.org/10.1007/BF00709654
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00709654