Abstract
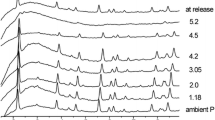
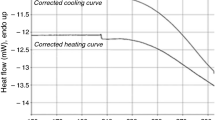
A high-pressure phase of the clathrate hydrate of tetrahydrofuran was prepared by freezing a liquid phase of overall composition THF · 7 H2O under a pressure of 3.0 kbar, or by pressurizing the solid structure II THF hydrate of 255K to 3.4 kbar. Unfortunately, the products recovered at 77K were always mixed phase materials as shown by X-ray powder diffraction. A number of diffraction lines could be indexed in terms of the cubic structure I hydrate with a slightly expanded lattice parameter, 12.08 Å, giving some support to Dyadin's idea that the high pressure phase transition involves a conversion of Structure II to Structure I. Other phases observed in the recovered product include Ice IX and amorphous materials. The reversion of the high pressure sample to the structure II hydrate was followed by differential scanning calorimetry. At ambient pressure, the high pressure sample converts slowly back to Structure II hydrate event at 77K.
Similar content being viewed by others
References
D.W. Davidson:Water, A Comprehensive Series, Ed. F. Franks, Plenum Press, N.Y., 1973, Vol. 2.
D.W. Davidson and J.A. Ripmeester:Inclusion Compounds, Ed. J.L. Atwood, J.E.D. Davies and D.D. MacNicol, Academic Press, London, 1984, Vol. 3.
J.A. Ripmeester, C.I. Ratcliffe, J.S. Tse and B.M. Powell:Nature 325, 135 (1987).
J.A. Ripmeester and C.I. Ratcliffe:J. Phys. Chem. 94, 8773 (1990).
O. Mishima, L.D. Calvert and E. Whalley:Nature,310, 393 (1984).
Y.P. Handa, J.S. Tse, D.D. Klug and E. Whalley:J. Chem. Phys. 94, 623 (1991).
S.R. Gough and D.W. Davidson:Can. J. Chem. 49, 2691 (1971).
R.G. Ross and P. Anderson:Can. J. Chem. 60, 881 (1982).
Y.A. Dyadin, P.N. Kuznetzov, I.I. Yakovlev and A.V. Pirinova:Dokl. Akad. Nauk. SSSR 208, 103 (1973).
Y.A. Dyadin, F.V. Zhurko, T.V. Mikina and R.K. Udachin:J. Incl. Phernom. 9, 37 (1990).
Y.A. Dyadin, F.V. Zhurko, I.V. Bondaryuk and G.O. Zhurko:J. Incl. Phenom. 10, 39 (1991).
D.D. Klug, O. Mishima and E. Whalley:J. Chem. Phys. 86, 5323 (1987).
J.A. Ripmeester, C.I. Ratcliffe and D.D. Klug:J. Chem. Phys. 96, 8503 (1992).
Y.P. Handa, D.D. Klug and E. Whalley:Can. J. Chem. 66, 919 (1988).
Author information
Authors and Affiliations
Additional information
NRCC No. 35786.
Rights and permissions
About this article
Cite this article
Zakrzewski, M., Klug, D.D. & Ripmeester, J.A. On the pressure-induced phase transformation in the structure II clathrate hydrate of tetrahydrofuran. J Incl Phenom Macrocycl Chem 17, 237–247 (1994). https://doi.org/10.1007/BF00708783
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00708783