Abstract
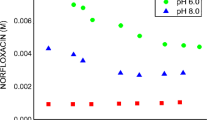
Solid combinations of naproxen with amorphous hydroxypropyl derivatives of α-, β-, and γ-cyclodextrin with an average substitution degree per anhydroglucose unit of 0.6 were investigated for thermal behaviour (differential scanning calorimetry), drug crystallinity (X-ray diffractometry), and dissolution rate (dispersed amount and rotating disc methods). Phase-solubility analysis and computer-aided molecular modelling were carried out to study the inclusion complexation of naproxen with hydroxypropyl cyclodextrins. The cavity size of the host is a selective factor for the solubilizing effect, complexing ability, and dissolution rate enhancement on naproxen, hydroxypropyl β-cyclodextrin being markedly the most effective derivative. No relationship was found between the decrease in crystallinity of the drug dispersed in the amorphous carrier matrix and the geometrical features of the cyclodextrin macrocycle.
Similar content being viewed by others
References
G. P. Bettinetti, P. Mura, A. Liguori, G. Bramanti, and F. Giordano:II Farmaco 44, 195 (1989).
D. Duchêne and D. Wouessidijewe:Pharm. Techn. In. 2, 21 (1990).
G. P. Bettinetti, P. Mura, F. Melani, and F. Giordano:Minutes 5th Int. Symp. Cyclodextrins (Ed. D. Duchêne, Ed. de Santé, Paris), pp. 239–242 (1990).
G. P. Bettinetti, A. Gazzaniga, P. Mura, F. Giordano, and M. Setti:Drug Dev. Ind. Pharm. 18, 39 (1992).
G. P. Bettinetti, P. Mura, F. Melani, and F. Giordano:Proc. 7th Intern. Cyclodextrins Symp. (Acad. Soc. Japan, Tokyo), pp. 455–458 (1994).
P. Mura, G. P. Bettinetti, F. Melani, and A. Manderioli:Eur. J. Pharm. Sci. in press (1995).
S. Brunauer, T. H. Emmett, and E. Teller:J. Am. Chem. Soc. 60, 309a (1938).
Byosim Technologies, 9685 Scranton Road, S. Diego, CA 92121-2777.
G. P. Bettinetti, F. Melani, P. Mura, R. Monnanni, and F. Giordano:J. Pharm. Sci. 80, 1162 (1991).
G. P. Bettinetti, P. Mura, F. Giordano, and M. Setti:Thermochim. Acta 199, 165 (1991).
K. A. Khan:J. Pharm. Pharmacol. 27, 48 (1975).
J. Blanco, J. L. Vila-Jato, F. Otero, and S. Anguiano:Drug Dev. Ind. Pharm. 17, 943 (1991).
O. I. Corrigan and C. T. Stanley:J. Pharm. Pharmacol. 34, 621 (1982).
R. J. Bergeron, D. M. Pillor, G. Gibeily, and W. P. Roberts:Bioorg. Chem. 7, 263 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Melani, F., Bettinetti, G.P., Mura, P. et al. Interaction of naproxen with α-, β-, and γ-hydroxypropyl cyclodextrins in solution and in the solid state. J Incl Phenom Macrocycl Chem 22, 131–143 (1995). https://doi.org/10.1007/BF00707688
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00707688