Summary
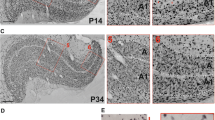
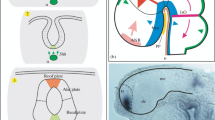
The sequence of development of cell layers in the neocortex of the tammar has been followed from 24 days gestation to 213 days postnatal. The tammar is born at 27 days gestation and the major period of its development occurs during the subsequent 250 days, most of this time being spent within the pouch. Although the pattern of differentiation of the cell layers appears to resemble that described for many Eutherian mammals, the neocortex is at an embryonic 2 layered stage at birth and a cortical plate is not present throughout the telencephalon until 10–15 days postnatal. A transient subplate zone, presenting a characteristic appearance with widely spaced rows of cells aligned parallel to the cortical surface, develops between 20 and 70 days postnatal, but no secondary proliferative region is seen in the subventricular zone of the dorso-lateral wall.
Preliminary experiments with (3H)-thymidine injections indicate that the cortical plate follows the “inside-out” pattern of development described in many Eutherian mammals and that the oldest neurons are found in the parallel cell rows of the subplate zone. The importance of the late differentiation of the neocortex in relation to the time of birth and the resulting usefulness of the tammar as an experimental model of cortical development is discussed.
Similar content being viewed by others
References
Abbie AA (1937) Some observations of the major subdivisions of the marsupialia with especial reference to the position of the Peramelidae and Caenolestidae. J Anat 71:429–436
Abbie AA (1939) The origin of the corpus callosum and the fate of structures related to it. J Comp Neurol 71:9–44
Altman J (1963) Autoradiographic investigation of cell proliferation in the brain of rats and cats. Anat Rec 145:573–591
Altman J (1966) Proliferation and migration of undifferentiated precursor cells in the rat during postnatal gliogenesis. J Exp Neurol 16:263–278
Angevine JB Jnr, Sidman RL (1961) Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 192:766–768
Benevento LA (1968) Organization of the visual cortex in the opossum. Anat Rec 160:313 (abst)
Benevento LA, Ebner FF (1971) The areas and layers of corticocortical terminations in the visual cortex of the Virginia opossum. J Comp Neurol 141:157–190
Berry M, Rogers AW (1965) The migration of neuroblasts in the developing cerebral cortex. J Anat (Lond) 99:69–109
Blakemore WF, Jolly RD (1972) The subependymal plate and associated ependyma in the dog. An ultrastructural study. J Neurocytol 1:69–84
Boulder Committee (1970) Embryonic vertebrate central nervous system: revised terminology. Anat Rec 166:257–261
Cavanagh ME, Møllgard K (1985) An immunocytochemical study of the distribution of some plasma proteins within the developing forebrain of the pig, with special reference to the neocortex. Dev Brain Res 17:183–194
Ebner FF (1969) A comparison of primitive forebrain organization of Metatherian and Eutherian mammals. Ann NY Acad Sci 196:241–257
Gray PA (1924) The cortical lamination pattern of the opossumDidelphys virginiana. J Comp Neurol 37:221–263
Heath CJ, Jones EG (1971) Interhemispheric pathways in the absence of a corpus callosum. J Anat 109:253–270
Jacobson M (1978) Developmental neurobiology. Plenum Press, New York
Johnson JL Jnr (1977) In: Hunsaker D (ed) The biology of the marsupials. II. Acad Press NY Chap 4, pp 159–264
Kluver H, Barrera E (1953) A method for the combined staining of cells and fibres in the nervous system. J Neuropathol Exp Neurol 12:400–403
Kostovic I, Molliver ME (1974) A new interpretation of the laminar development of cerebral cortex synaptogenesis in different layers of the neopallium in the human foetus. Anat Rec 178:395
Kunze D, Putman S, Manning JW (1968) Transcortical striate connections in the opossum. J Comp Neurol 132:463–468
Langworthy OR (1927) Correlated physiological and morphological studies on the development of electrically responsive areas in the cerebral cortex of the opossum. Contributions to Embryology, Carnegie Inst 19:149–173
Langworthy OR (1928) The behaviour of pouch young opossums correlated with myelinization of tracts of the nervous system. J Comp Neurol 46:201–247
Lende RA (1963) Motor representation in the cerebral cortex of the opossum (Didelphys virginiana). J Comp Neurol 121:405–415
Lende RA (1963) Cerebral cortex; a sensorimotor amalgam in the marsupialia. Science 141:730–732
Lende RA (1969) A comparative approach to the neocortex: localization in monotremes, marsupials and insectivores. Ann NY Acad Sci 196:262–276
Leuba G, Hermann D, Rabinowicz T (1978) Postnatal development of the mouse cerebral neocortex. III Some dynamical aspects. J Hirnforsch 19:301–312
Lewis PD (1968a) A quantitative study in cell proliferation in the subependymal layer of the adult rat brain. Exp Neurol 20:203–207
Lewis PD (1968b) Mitotic activity in the primate subependymal layer and the genesis of gliomas. Nature 217:974–975
Loo YT (1930) The forebrain of the opossumDidelphys virginiana: Part I Gross anatomy. J Comp Neurol 51:13–64
Luskin MB, Schatz CJ (1985) Studies on the earliest generated cells of the cat visual cortex: cogeneration of subplate and marginal zones. J Neurosciences 5:1062–1075
Macchi C (1951) The ontogenetic development of the olfactory telencephalon in man. J Comp Neurol 95:245–305
Magalhaes-Castro B, Saraiva PES (1971) Sensory and motor representation in the cerebral cortex of the marsupialDidelphys azarae azarae. Brain Res 34:291–299
Marin-Padilla M (1978) Dual origin of the mammalian neocortex and evolution of the cortical plate. Anat Embryol 152:109–126
Martin GF (1967) Interneocortical connections in the opossumDidelphys virginiana. Anat Rec 157:607–616
Møllgard K, Jacobsen M (1984) Immunohistochemical identification of some plasma proteins in human embryonic and fetal forebrain with particular reference to the development of the neocortex. Dev Brain Res 13:49–63
Møllgard K, Reynolds ML, Jacobsen M, Dziegielewska KM, Saunders NR (1984) Differential immunocytochemical staining for fetuin and transferrin in the developing cortical plate. J Neurocytol 13:497–502
Molliver ME, Van der Loos H (1970) The ontogenesis of cortical circuitry: the spatial distribution of synapses in somesthetic cortex of newborn dog. Ergeb Anat Entwickl-Gesch 42:1–54
Molliver ME, Kostovic I, Van der Loos H (1973) The development of synapses in the cerebral cortex of the human fetus. Brain Res 50:403–407
Morest DK (1969) The growth of dendrites in the mammalian brain. Z Anat Entwickl-Gesch 128:290–317
Morest DK (1970) A study of neurogensis in the forebrain of the opossum pouch young. Z Anat Entwickl-Gesch 130:265–305
Privat A, Leblond CP (1972) the subependymal layer and neighbouring region in the brain of the young rat. J Comp Neurol 146:277–302
Raedler E, Raedler A, Feldhaus S (1980) Dynamical aspects of neocortical histogenesis in the rat. Anat Embryol 158:253–269
Renfree M, Holt AB, Green JW, Carr JP, Cheek DB (1982) Ontogeny of the brain in a marsupial (Macropus eugenii) through pouch life. Brain Behav Evol 20:57–71
Reynolds ML, Møllgard K (1985) The distribution of plasma proteins in the neocortex and early allocortex of the developing sheep brain. Anat Embryol 171:41–60
Rockel AJ, Heath CJ, Jones EG (1972) Afferent connections to the thalamus in the marsupial phalanger and the question of sensory convergence in the thalamus of primitive mammals. J Comp Neurol 145:105–120
Sidman RL (1970) Autoradiographic methods and principles for study of the nervous system with3H-thymidine. In: Ebbesson SOE, Nauta WJ (eds). Contemporary research techniques of neuroanatomy. Springer NY. p 252–274
Smart IHM (1961) The subependymal layer of the mouse brain and its cell production as shown by radioautography after thymidine — H3 injection. J Comp Neurol 116:325–347
Stensaas LJ (1967) The development of the hippocampal and dorsolateral pallial regions of the cerebral hemisphere in fetal rabbits. II. Twenty millimetre stage, neuroblast morphology. J Comp Neurol 129:71–84
Tyndale-Biscoe CH, Hinds LA (1984) Seasonal patterns of circulating progesterone and prolactin and response to bromocriptine in the female tammarMacropus eugenii. Gen Comp Endocrinol 53:58–68
Walsh TM, Ebner FF (1968) Organization of the somatic — motor cortex in the opossum. Anat Rec 160:446
Walsh TM, Ebner FF (1970) The cytoarchitecture of somatic sensory — motor cortex in the opossumDidelphys marsupialias virginiana; a Golgi study. J Anat 107:1–18
Ward JW (1954) The development of the cortico-spinal tract in the pouch young of the virginia opossumDidelphys virginiana. J Comp Neurol 101:483–494
Weed LH, Langworthy OR (1925) A development study of excitatory areas in the cerebral cortex of the opossum. Am J Physiol 72:8–24
Wye-Dvorak J (1984) Postnatal development of primary visual projections in the tammar wallaby (Macropus eugenii). J Comp Neurol 228:491–508
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reynolds, M.L., Cavanagh, M.E., Dziegielewska, K.M. et al. Postnatal development of the telencephalon of the tammar wallaby (Macropus eugenii). Anat Embryol 173, 81–94 (1985). https://doi.org/10.1007/BF00707306
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00707306