Abstract
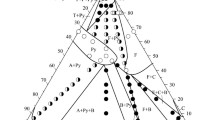
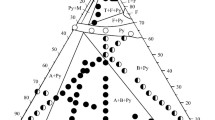
A phase diagram of the [Zn(MePy)2(NCS)2]-MePy binary system has been studied by DTA and solubility methods. Two compounds melting incongruently (at 63 and 57°C) have been discovered in the system. They have been obtained as separate phases with crystals of different shape (needles and octahedra). Their composition has been determined by analytical methods and verified by X-ray structural analysis: [Zn(MePy)4(NCS)2]·0.67 MePy·xH2O, wherex depends on the synthesis conditions, and [Zn(MePy)4(NCS)2]·MePy, respectively.
The preliminary X-ray study of the first compound (at −100°C) has shown it to be isostructural with the known clathrates of the common formula [M(MePy)4(NCS)2]·0.67MePy·xH2O, where M=Cu, Mn, Mg andx=0–0.33. The unit cell parameters are as follows:a=27.20(1),c=11.202(4) Å, space group\(R\bar 3\),Z=9.
The X-ray study of [Zn(MePy)4(NCS)2]·MePy (at−50°C) has shown it to be analogous to the ‘organic zeolite’ β-phase with the guest MePy molecules located in the channels formed by molecular packing of the [Zn(MePy)4(NCS)2]host. The cell is tetragonal, the space groupI41/a,a=16.845(6),c=23.496(7) Å,V=6667(4) Å3,Z=8,D calc=1.289 g cm−3,R=0.074. The zinc cation is surrounded by a slightly irregular octahedron of six nitrogen atoms of the MePy and NCS ligands. The crystallisation field of the host [Zn(MePy)4(NCS)2] complex in the temperature range concerned is absent in the phase diagram. It suggests contact stabilization of the [Zn(MePy)4(NCS)2] molecule by the guest in the clathrates.
Similar content being viewed by others
References
J. Lipkowski: inInclusion Compounds, Vol. 1 (Ed.) J.L. Atwood, J.E.D. Davies and D.D. MacNicol, pp 59–103, Academic Press, London (1984).
Yu.A. Dyadin and N.V. Kislykh:Mendeleev Commun. 4, 134 (1991).
D. Biddiscombe, E. Coulson, R. Hendley, and E. Herington:J. Chem. Soc. 6, 1957 (1954).
W.D. Schaeffer, W.5. Dorsey, D.A. Skinner, and C.G. Christian:J. Am. Chem. Soc. 79 5870 (1957).
5.A. Allison and R.M. Barrer:J. Chem. Soc. A. 1717 (1969).
E.A. Ukraintseva, N.V. Kislykh, Yu.A. Dyadin, D.V. Soldatov, and V.A. Logvinenko:Sibirskij Khimicheskij Zhurnal, vyp2, 50 (1993).
N.V. Kislykh, Yu.A. Dyadin, N.V. Pervukhina, I.V. Davydowa, and N.V. Podberezskaya:Izv. Sib. Otd. Akad. Nauk. SSSR, Ser. Khim. Nauk 3, 76 (1989);Chem. Abstr. 111, 141646n (1989).
G.M. Sheldrick, SHELX-86: inCrystallographic Computing 3 (Eds., G.M. Sheldrick, O. Krueger, and R. Goddard), pp. 175–189, Oxford University Press, Oxford (1985).
G.M. Sheldrick, SHELX-76:Program for Crystal Structure Determination, University of Cambridge, England (1976).
N. Walker and D. Stuart:Acta Crystallogr. A39, 158 (1983).
M. Nardelli:Comput. Chem. 7, 95 (1983).
Yu.A. Dyadin, N.V. Kislykh, G.N. Chekhova, N.V. Podberezskaya, N.V. Pervukhina, V.A. Logvinenko, and I.M. Oglezneva:J. Incl. Phenom. 2, 333 (1984).
E.A. Ukraintseva, N.V. Kislykh, Yu.A. Dyadin, and V.A. Logvinenko:Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk 5, 69 (1989);Chem. Abstr. 112, 171059m (1990).
Yu.A. Dyadin, N.V. Kislykh, G.N. Chehova, V.A. Logvinenko, I.M. Oglezneva, C.B. Erenburg, and L.N. Mazalov:Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk 2, 58 (1986);Chem. Abstr. 104, 214061k (1986).
J. Lipkowski and S. Majchrzak:Rocz Chem. 49, 1655 (1975).
J. Lipkowski:J. Coord. Chem. 22, 153 (1990).
N.V. Pervukhina, N.V. Podberezskaya, I.V. Davydova, N.V. Kislykh, and Yu.A. Dyadin:J. Incl. Phenom. 13, 9 (1992).
C.K. Johnson, ORTEP:Report ORNL-3794, Oak Ridge National Laboratory, Tennessee (1965).
W.L. Steffen and G.J. Palenik:Inorg. Chem. 16, 1119 (1977).
H. Lynton and M.C. Sears:Can. J. Chem. 49, 3418 (1971).
I. Agrell:Acta Chem. Scand. 24, 1247 (1970).
A.F. Cameron, D.W. Taylor, and R.H. Nuttall:J. Chem. Soc. A., 3402 (1971).
P.J.F. Le Querler, M.M. Bore, and A. Leclaire:Acta Crystallogr. B33, 2299 (1977).
M.A. Hitchman, R. Thomas, B.W. Skelton, and A.H. White:J. Chem. Soc., Dalton Trans., 2273 (1983).
A.F. Cameron, D.W. Taylor, and R.H. Nuttall:J. Chem. Soc., Dalton Trans. 1603 (1972).
J. Lipkowski:J. Mol. Struct. 75, 13 (1981).
J. Lipkowski, K. Suwińska, G.D. Andreetti, and K.J. Stadnicka:J. Mol. Struct. 75, 101 (1981).
Author information
Authors and Affiliations
Additional information
Supplementary Data relevant to this paper have been deposited with the British Library at Boston Spa, Wetherby, West Yorkshire, U.K. as Supplementary Publication No. SUP 82166 (15 pages).
Rights and permissions
About this article
Cite this article
Lipkowski, J., Soldatov, D.V., Kislykh, N.V. et al. Phase and structural study of clathrate formation in the [Zn(MePy)2(NCS)2]-MePy system (MePy=4-methylpyridine). J Incl Phenom Macrocycl Chem 17, 305–316 (1994). https://doi.org/10.1007/BF00707126
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00707126