Abstract
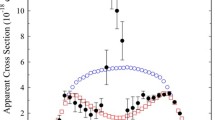
The kinetic energy dependence of collision-induced dissociation (CID) of dicobalt ion (Co +2 ) with He, Ar, and Xe has been investigated using guided ion-beam mass spectrometry. The change in efficiency of CID as the target gas is changed is in general agreement with previous CID studies of other systems: the cross section with Ar is ∼0.5 that with Xe, and no product ions are found with He. By varying the conditions under which the reactant ions are formed, the degree of internal excitation of the dicobalt ions is changed. The internal energies can be characterized by a Maxwell-Boltzmann distribution. We find that CID and reactions with O2 and CO are very sensitive to Co +2 internal energy. The bond-dissociation energy derived from this work is Do(Co +2 )=2.75±0.10 eV (63.4±2.3 kcal/mol). The Co +2 results are compared with a previous study of Fe +2 .
Similar content being viewed by others
References
M. D. Morse (1986).Chem. Rev. 86, 1049.
J. M. Alford, F. D. Weiss, R. T. Laaksonen, and R. E. Smalley (1986).J. Phys. Chem. 90, 4480.
J. L. Elkind, F. D. Weiss, J. M. Alford, R. T. Laaksonen, and R. E. Smalley (1988).J. Chem. Phys. 88, 5215.
M. E. Geusic, M. D. Morse, S. C. O'Brien, and R. E. Smalley (1985).Rev. Sci. Instrum. 56, 2123.
L. Hanley, S. A. Ruatta, and S. L. Anderson (1987).J. Chem. Phys. 87, 260.
L. Hanley and S. L. Anderson (1985).Chem. Phys. Lett. 122, 410.
L. Hanley and S. L. Anderson (1986).Chem. Phys. Lett. 129, 429.
D. B. Jacobson and B. S. Freiser (1984).J. Am. Chem. Soc. 106, 5351.
D. B. Jacobson and B. S. Freiser (1986).J. Am. Chem. Soc. 108, 27.
K. Ervin, S. K. Loh, N. Aristov, and P. B. Armentrout (1983).J. Phys. Chem. 87, 3593.
S. C. Richtsmeier, E. K. Parks, K. Liu, L. G. Pobo, and S. J. Riley (1985).J. Chem. Phys. 82, 3659.
M. R. Zakin, R. O. Brickman, D. M. Cox, and A. Kaldor (1988).J. Chem. Phys. 88, 5943.
S. K. Loh, D. A. Hales, and P. B. Armentrout (1986).Chem. Phys. Lett. 129, 527.
S. K. Loh, L. Lian, D. A. Hales, and P. B. Armentrout (1988).J. Phys. Chem. 92, 4009.
M. R. Zakin, R. O. Brickman, D. M. Cox, and A. Kaldor (1988).J. Chem. Phys. 88, 3555.
L.-S. Zheng, P. J. Brucat, C. L. Pettiette, S. Yang, and R. E. Smalley (1985).J. Chem. Phys. 83, 4273.
P. J. Brucat, L.-S. Zheng, C. L. Pettiette, S. Yang, and R. E. Smalley (1986).J. Chem. Phys. 84, 3078.
P. J. Brucat, C. L. Pettiette, S. Yang, L.-S. Zheng, M. J. Craycraft, and R. E. Smalley (1986).J. Chem. Phys. 85, 4747.
K. M. Ervin and P. B. Armentrout (1985).J. Chem. Phys. 83, 166.
S. K. Loh, D. A. Hales, L. Lian, and P. B. Armentrout (1989).J. Chem. Phys. 90, 5466.
N. R. Daly (1959).Rev. Sci. Instrum. 31, 264.
R. E. Winters and R. W. Kiser (1965).J. Phys. Chem. 69, 1618.
C. Rebick and R. D. Levine (1973).Chem. Phys. 58, 3942.
E. K. Parks, A. Wagner, and S. Wexler (1973).J. Chem. Phys. 58, 5502.
R. D. Levine and R. B. Bernstein (1971).Chem. Phys. Lett. 11, 552.
T. F. Moran and D. C. Fullerton (1971).J. Chem. Phys. 54, 5231.
W. B. Maier II (1964).J. Chem. Phys. 41, 2174.
R. Viswanathan, L. M. Raff, and D. L. Thompson (1983).J. Chem. Phys. 79, 2857.
N. Aristov and P. B. Armentrout (1986).J. Phys. Chem. 90, 5135.
S. K. Loh, L. Lian, and P. B. Armentrout (1989).J. Am. Chem. Soc. 111, 3167.
C. Lifshitz, R. L. C. Wu, T. O. Tiernan, and D. T. Terwilliger (1978).J. Chem. Phys. 68, 247.
E. W. Rothe and R. B. Bernstein (1959).J. Chem. Phys. 31, 1619.
D. A. Hales, L. Lian, and P. B. Armentrout (1990, in preparation).
V. L. Talrose, P. S. Vinogradov, and I. K. Larin,in M. T. Bowers (ed.),Gas Phase Ion Chemistry, Vol. 1 (Academic Press, New York, 1979), p. 305.
S. G. Lias, J. E. Bartmess, J. F. Liebman, J. L. Holmes, R. D. Levin, and W. G. Mallard (1988).J. Phys. Chem. Ref. Data 17 (suppl. 1), 599.
A. Kant and B. Strauss (1964).J. Chem. Phys. 41, 3806.
I. Shim and K. Gingerich (1983).J. Chem. Phys. 78, 5693.
D. G. Leopold and W. C. Lineberger (1986).J. Chem. Phys. 85, 51.
E. A. Rohlfing, D. M. Cox, and A. Kaldor (1984).J. Chem. Phys. 81, 3846.
J. Sugar and C. Corliss (1985).J. Phys. Chem. Ref. Data 14 (suppl. 2), 1.
K. Hilpert (1979).Ber. Bunsenges. Phys. Chem. 83, 161.
M. D. Morse, G. P. Hansen, P. R. R. Langridge-Smith, L.-S. Zheng, M. E. Geusic, D. L. Michalopoulos, and R. E. Smalley (1984).J. Chem. Phys. 80, 5400.
D. B. Jacobson and B. S. Freiser (1984).J. Am. Chem. Soc. 106, 4623.
P. B. Armentrout (1986).Proc. SPIE 620, 38.
M. F. Jarrold, A. J. Illies, and M. T. Bowers (1985).J. Am. Chem. Soc. 107, 7339.
D. R. Bidnosti and N. S. McIntyre (1970).Can. J. Chem. 48, 593.
R. J. McKinney and D. A. Pensak (1979).Inorg. Chem. 18, 3413.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hales, D.A., Armentrout, P.B. Effect of internal excitation on the collision-induced dissociation and reactivity of Co +2 . J Clust Sci 1, 127–142 (1990). https://doi.org/10.1007/BF00703589
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00703589