Abstract
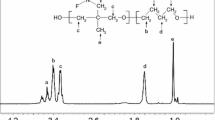
Thermal decomposition of poly[oxybis(dimethylsilylene)] having chains terminated with trimethylsiloxy groups was studied by thermogravimetry, pyrolysis-mass spectrometry, and infrared spectroscopy. The polymer is thermally less stable than poly(dimethylsiloxane). Depolymerization occurs at temperatures of 250–350°C, although this process also takes place at lower temperatures. The depolymerization produces cyclic oligomers of general formula [(Me2Si)2O] n , with predominant formation of the oligomern=2. The depolymerization is accompanied by processes which are referred to as restructurization because they change the structure of the polymer backbone. Decomposition may lead also to the formation of branching points. The shape of the thermograms taken under isothermal conditions is in agreement with an unzipping mechanism for depolymerization involving random initiation. Excluding the short initial period of the process, the unzipping is terminated at a restructurization point. A low activation energy points to initiation induced by electron transfer, presumably involving traces of contaminant. At higher temperatures, 350–600°C, loss of organic parts of the polymer takes place along with further restructurization. At higher temperatures the polymer was also found to undergo easily oxygenation of its backbone with atmospheric oxygen, which leads to the formation of siloxane groups.
Similar content being viewed by others
References
T. H. Thomas and T. C. Kendrick,J. Polym. Sci. A2 7, 537 (1969).
N. Grassie and J. G. McFarlane,Eur. Polym. J. 14, 857 (1978).
M. Zeldin, Bo-Rong Qian, and Sung J. Choi,J. Polym. Sci. Polym. Chem. Ed. 21, 1361 (1983).
A. Ballistreri, D. Garozzo, and G. Montaudo,Macromolecules 17, 1312 (1984).
T. C. Kendrick, B. Parbhoo, and J. W. White, inChemistry of Organic Silicon Compounds, Part 2, S. Patai and Z. Rappaport, eds. (J. Wiley and Sons, Chichester, 1989), Vol. 2, p. 1319.
R. West, inChemistry of Organic Silicon Compounds, S. Patai and Z. Rappaport, eds. (J. Wiley and Sons, New York, 1989), Part 2, p. 1207.
S. Yajima,Ceram. Bull. 62, 893 (1983).
P. Trefonas, inEncyclopedia of Polymer Science and Engineering, H. F. Mark, N. M. Bikales, C. G. Overberger, and G. Manges, eds. (J. Wiley and Sons, New York, 1988), Vol. 13, p. 162.
J. Chojnowski, J. Kurjata, and S. Rubinsztajn,Makromol. Chem. Rapid Commun. 15, 469 (1988).
J. Chojnowski, J. Kurjata, S. Rubinsztajn, and M. Scibiorek, inFrontiers of Organosilicon Chemistry, A. R. Bassindale and P. P. Gaspar, eds. (Royal Society of Chemistry, Cambridge, 1991), p. 70.
M. R. Litzow and T. R. Spalding, (Elsevier, Amsterdam, 1973), p. 285.
A. Ballistreri, D. Garozzo, and G. Montaudo,Macromolecules 17, 1312 (1984).
J. H. Flynn and L. A. Wall,J. Res. Natl. Bur. Stand. A Phys. Chem. 70A, 487 (1966).
W. Patnode and D. F. Wilcock,J. Am. Chem. Soc. 68, 358 (1946).
C. W. Lewis,J. Polym. Sci. 37, 425 (1959).
M. Kucera, J. Lanikova, and M. Jelinek,J. Polym. Sci. 53, 301 (1961).
J. M. Cox, B. A. Wright, and W. W. Wright,J. Appl. Polym. Sci. 8, 2951 (1964).
V. V. Rode, M. A. Verkhotin, and S. R. Rafikiv,Vysokomol. Soedin. A11, 1529 (1969).
K. A. Andrianov, V. S. Papkov, G. L. Slonimskii, A. A. Zhdanov, and S. Ye. Yakushkina,Vysokomol. Soedin. A11, 2030 (1969).
G. B. Tanny and L. E. St. Pierre,J. Polym. Sci. B9, 863 (1971).
M. Blazso, G. Garzo, and T. Szekely,Chromatographia 5, 489 (1972).
M. Zeldin, G. P. Rajendran, and M. S. Beder,Polym. Mater. Sci. Eng 49, 274 (1983).
D. W. Kang, G. P. Rajendran, and M. Zeldin,J. Polym. Sci. Polym. Chem. Ed. 24, 1085 (1986).
R. West, inComprehensive Organometallic Chemistry, G. Wilkinson, G. Stone, and E. W. Abel, eds. (Pergamon Press, Oxford, 1982), Vol. 2, p. 365.
R. D. Miller and J. Michl,Chem. Rev. 89, 1359 (1989).
J. M. Funt, D. Parekh, J. H. Magill, and Y. T. Shah,J. Polym. Sci. Polym. Chem. Ed. 13, 2181 (1975).
W. R. Schmidt, L. V. Interrante, R. H. Doremus, T. K. Trout, P. S. Marchetti, and G. E. Maciel,Chem. Mater. 3, 257 (1991).
T. R. Crompton,Chemical Analysis of Organometallic Compounds (Academic Press, London, 1974), Vol. 2, p. 91.
H. Marsmann, inNMR Basic Principles and Progress, P. Diehl, E. Fluck, and R. Kosfeld, eds. (Springer Verlag, Berlin, 1981), Vol. 17, p. 65.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chojnowski, J., Kurjata, J., Rubinsztajn, S. et al. Thermal decomposition of poly(tetramethyloxydisilaethylene). J Inorg Organomet Polym 2, 387–404 (1992). https://doi.org/10.1007/BF00701113
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00701113