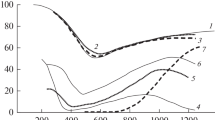
Conclusions
-
1.
The temperature dependence of the carburizing rate coeffcient in H2−CH4−H2O mixtures diluted with nitrogen is described by the Arrhenius equation K=K·e−Q/RT.
The activation energy of this proces depends on carbon content in the steel and it may be determined from the relationship Q=(55000+e11C)·4.187 kJ/mole (where C is carbon content in the steel, %).
-
2.
The mass transfer coefficient during carburizing in H2−CH4−H2O mixtures diluted with nitrogen decreases with an increase in carbon content in the foil.
-
3.
The specific flow of carbon into the metal depends to a considerable degree on carburizing temperature, methane content in the mixture, and carbon content in the steel.
-
4.
A reduction in carburizing intensity with an increase in carbon content in the steel may be connected with development of pyrocarbon nuclei and a reduction in the number of active centers of the foil surface.
Similar content being viewed by others
Literature cited
V. B. Skakal'skii, A. A. Skvortsov, and N. A. Titov, "Industrial nitrogen as a basis of universal controlled atmosphere," Metalloved. Term. Obrab. Met., No. 5, 31–33 (1978).
T. Ellison, I. Taylor, and G. Boggley, Iron Steel Eng.,57, No. 11, 51–57 (1980).
M. Kostelitz, Heat Treat, 79, Proc. Int. Conf. (Birmingham, 1979), London (1980).
P. A. Tesner, Formation of Carbon from Gas Phase Hydrocarbons [in Russian], Khimiya, Moscow (1972).
B. M. Éstrin, "Carburizing in a CH4−H2−H2O gas mixture diluted with nitrogen," Metalloved. Term. Obrab. Met., No. 10, 31–35 (1979).
B. M. Éstrin, O. S. Mnushkin, M. A. Shkol'nikov, and V. I. Okun', "Equipment for chemicothermal treatment of steel in oxygen-free mixtures based on nitrogen," Chermetinformatsiya, Bulletin No. 3, 60–61, Moscow.
P. A. Tesner, N. B. Golvina, A. E. Gorodetskii, et al. "Kinetics of pyrocarbon formation from CH4," Khim. Tverd. Topliva, No. 1, 129–135 (1976).
M. M. Leonidova, L. A. Shvartsman, and L. A. Shul'ts Physicochemical Bases of the Reaction of Metals with Controlled Atmospheres [in Russian], Metallurgiya, Moscow (1980).
A. A. Popov, Thermodynamic Bases of Chemicothermal Treatment of Steel [in Russian], Metallurgizdat, Moscow (1962).
H. I. Grabke, Berichte der Bunsen-gesselschaft,69, No. 5 409–414 (1965).
V. A. Munts and A. P. Baskakova, "Kinetic relationships of steel carburization," Metalloved. Term. Obrab. Met., No. 5 48–51 (1980).
P. Martin Abvarez et al., Rev. Met. CENIM,13, No. 5, 270–274 (1977).
T. V. Tekunova and P. A. Tesner, "Kinetic formation of pyrocarbon from CH4 on Pt, Ni, Mo, W," Katal. Konversiya Uglevodorodov, No. 3, 29–31 (1978).
Additional information
Central Institute of Power Systems and Ferrous Metallurgy. Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 12, pp. 7–11, December, 1985.
Rights and permissions
About this article
Cite this article
Éstrin, B.M., Mnushkin, O.S. & Shkol'nikov, M.A. Kinetics of carburizing in a nitrogen-hydrogen atmosphere with addition of pure methane. Met Sci Heat Treat 27, 881–886 (1985). https://doi.org/10.1007/BF00700094
Issue Date:
DOI: https://doi.org/10.1007/BF00700094