Abstract
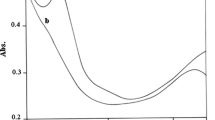
The mechanism of proton exchange between semiquinone neutral radicals 3,6-di-tert-butyl-2-hydroxyphenoxyl (1), 6-tert-butyl-3-chloro-2-hydroxy-4-triphenylmethylphenoxyl, and hydrochloric acid in toluene solutions has been studied. The rate of proton exchange with hydrochloric acid is less than that with acetic acid owing to the higher thermodynamic stability of the radical cation formed upon semiquinone radical protonation by hydrochloric acid. The formation of radical cations and their dimers has been proven by spectroscopy.
Similar content being viewed by others
References
A.I.Prokof'ev, N.N.Bubnov, S.P.Solodovnikov, I.S.Belostotskaya, V.V.Ershov, and M.I.Kabachnik,Izv.Akad.Nauk SSSR, Ser.Khim., 1974, 2467 [Bull.Acad.Sci.USSR.Div.Chem.Sci., 1974,23, No 11].
A.S.Masalimov, A.I.Prokof'ev, N.N.Bubnov, S.P.Solodovnikov, and M.I.Kabachnik,Izv.Akad.Nauk SSSR, Ser.Khim., 1977, 767 [Bull.Acad.Sci.USSR. Div.Chem.Sci., 1977,26, No 4].
A.Carrington and A.DMcLachlan,Introduction to Magnetic Resonance, Harper & Row Publishers, New York, Evanston and London, 1967
G.Denisov, S.Bureiko, N.Golubev, and K.Tokhadze,The Kinetics of the Exchange Processes of Proton Transport in Systems with Hydrogen Bonds, inMolecular Interactions. V.2, Ed.H.Batajczak, A Wiley-Interscience Publication, Chichester, New York, Brisbane, Toronto, 1984.
V.D.Pokhodenko, L.S.Degtyarev, V.G.Koshechko, and V.S.Kuts,Problemy Khimii Svobodnyh Radikalov (Problems of Chemistry of Free Radicals), Naukova Dumka, Kiev, 1984 (in Russian).
P.Sullivan and J.Bolton,Adv.Magn.Res., 1970,4, 39.
A.S.Masalimov, O.D.Kemalov, E.K.Zhumadilov, and A.I.Prokof'ev,Spektry EPR Ionnyh Par i Dimerov Semikhinonnyh Anion-radicalov s Kationami Shchelochnyh Metallov (ESR Spectra of Ion Pairs and Dimers of Semiquinone Radical Anions with Alkali Cations), inLuminescentsiya i Issledovaniye Parametrov Izlucheniya (Luminescence and Investigation of Emission Parameters), Proceedings of the Scientific Conference of the University at Karaganda, Karaganda, 1987 (in Russian).
R.Levingston and G.Zeldes,Issledovaniya Fotoliza v Zhidkostyah Metodom EPR (Investigations on Photolysis in Liquids by the ESR Method) inSvobodno-radikal'nye Sostoyaniya v Khimii (Free Radical States in Chemistry),Proceedings of the Scientific Conference on Free Radicals, Nauka, Novosibirsk, 1972 (in Russian).
A.S.Masalimov, S.N.Nikol'skii, A.V.Khodak, and A.I.Prokof'ev,Teor.Eksp.Khim., 1991, 184 [Theoret. Exp. Chem., 1991 (Engl.Transl.)].
A.S.Masalimov, S.N.Nikol'skii, and A.I.Prokof'ev,Issledovaniye Metodom EPR Kinetitcheskoi Kislotnosti 4-trifenilmetil-6-tret-butil-3-khlor-2-oksifenola (ESR Study of Kinetic Acidity of 6-tert-Butyl-3-chloro-2-hydroxy-4-tri-phenylmethylphenol) inFiziko-khimicheskiye Issledovaniya Stroeniya i Reaktsionnoi Sposobnosti Veshchestva (Physico-Chemical Studies of the Structure and Reactivity of a Substance), Proceedings of the Scientific Conference of the University at Karaganda, Karaganda, 1988 (in Russian).
Author information
Authors and Affiliations
Additional information
Translated fromIzvestiya Akademii Nauk. Seriya Khimicheskaya, No 1, pp. 84–87, January, 1993.
Rights and permissions
About this article
Cite this article
Masalimov, A.S., Melbardis, L.E. & Prokof'ev, A.I. Kinetics of proton exchange between semiquinone radicals and hydrochloric acid. Russ Chem Bull 42, 74–77 (1993). https://doi.org/10.1007/BF00699978
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00699978