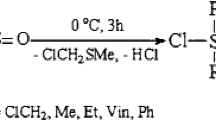Abstract
1,2,2-Trimethyldisilane-1,1,2-triol (1) is formed as an unstable intermediate upon hydrolysis of oligo(trimethyldisilanylsesquiazane). In the absence of trapping agents it undergoes rapid condensation to give ether-soluble poly(trimethyldisilanyloxane) which contains silanol groups. Treatment of the hydrolysis products with chlorotrimethylsilane in the presence of triethylamine affords trimethylsiloxy derivatives, (Me3SiO)2MeSiSiMe2(OSiMe3),5,6, and [Si2Me3O x (OSiMe3) y ] n . The isolation of these products indicates that disilanetriol1 readily undergoes condensation to form hydroxylcontaining six-membered rings and polysiloxanes. The condensation of compound1 in the presence of Me3SiOH has been studied. The ratio between the isomeric cyclosiloxanes5 and6 has been determined both by1H NMR spectroscopy and by a chemical method (chlorinolysis).
Similar content being viewed by others
References
K. A. Andrianov, inMetody elementoorganicheskoi khimii. Kremnii [Methods of Organometallic Chemistry. Silicon], Nauka, Moscow, 1968, 207 (in Russian).
M. F. Shostakovskii, I. A. Shikhiev, D. A. Kochkin, and V. I. Belyaev,Zh. Obshch. Khim., 1954,24, 2202 [J. Gen. Chem. USSR, 1954,24 (Engl. Transl.)].
U. G. Stolberg,Chem. Ber., 1963,96, 2798.
M. Kumada and K. Tamao,Adv. Organomet. Chem., 1968,6, 38.
C. G. Pitt,J. Am. Chem. Soc., 1967,91, 6613.
H. Gilman and R. L. Harrell,J. Organomet. Chem., 1966,5, 199.
V. V. Semenov, T. A. Chesnokova, and S. Ya. Khorshev,Zh. Obshch. Khim., 1991,61, 1195 [J. Gen. Chem. USSR. 1991,61 (Engl. Transl.)].
V. N. Alekseev,Kolichestvennyi analiz [Quantitative Analysis], Khimiya, Moscow, 1972, 245 (in Russian).
A. Weisssberger, E. Proskauer, J. Riddig, and E. Tups,Organic Solvents. Physical Properties and Methods of Purification, Interscience, New York, 1955.
F. M. Rappoport, A. A. Il'inskaya,Laboratornye metody polucheniya chistykh gazov [Laboratory Methods for Preparing Pure Gases], GNTI Khim. Lit., Moscow, 1963, 126 (in Russian).
V. V. Semenov and T. A. Chesnokova,Zh. Obshch. Khim., 1990,60, 150 [J. Gen. Chem. USSR, 1990,60 (Engl. Transl.)].
U. Scheim, K. Ruehlmann, H. Grosse-Ruyken, and A. Porzel,J. Organomet. Chem., 1986,314, 39.
K. A. Andrianov, inMetody elementoorganicheskoi khimii. Kremnii [Methods of Organometallic Chemistry. Silicon], Nauka, Moscow, 1968, 533 (in Russian).
Author information
Authors and Affiliations
Additional information
Translated fromIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 10, pp. 1788–1792, October, 1993.
Rights and permissions
About this article
Cite this article
Semenov, V.V., Artemicheva, S.B. & Kurskii, Y.A. 1,2,2-Trimethyldisilane-1,1,2-triol: intermediate formation and conversion to condensation products. Russ Chem Bull 42, 1713–1716 (1993). https://doi.org/10.1007/BF00697048
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00697048