Abstract
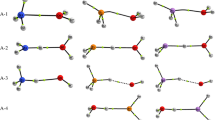
Quantum-chemical calculations (CNDO/2) of the theoretical relationship between the rate constants for the dimerization and self-protonation of radical anions show that dimer formation in the one-electron electroreduction of aromatic carboxylic acids (benzoic (1), 1-naphthoic (2), and 9-anthroic (3) is most probable for1. It is established that during the constant potential electrolysis (CPE) of1 a mixture of “head-to-tail” dimers is formed in the presence of 0.1M Bu4NClO4 (DMF). Their ratio depends on the amount of electricity passed through the solution. The CPE of 2 in the presence of 20 % H2O affords 1,4-dihydro-1-naphthoic acid in up to 70 % yield. The high yield (∼70 %) of 9,10-dihydro-9-anthroic acid during the CPE of 3 can be accounted for by the decomposition of the dimeric product followed by protonation of the anionic species.
Similar content being viewed by others
References
A. S. Mendkovich, O. Hammerich, T. Ya. Rubinskaya, and V. P. Gultyai,Acta Chem. Stand., 1991,45, 644.
V. P. Gultyai and A. S. Mendkovich,J. Electroanal. Chem., 1983,145, 201.
A. S. Mendkovich and V. P. Gultyai,Teoreticheskie osnovy khimii organicheskikh anion-radicalov [Theoretical Fundamentals of the Organic Radical Anion Chemistry], Nauka, Moscow, 1990, 152 (in Russian).
V. P. Gultyai, A. S. Mendkovich, and T. Ya. Rubinskaya,Izv. Akad. Nauk SSSR, Ser. Khim., 1987, 1576 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1987,36, 1455 (Engl. Transl.)].
V. P. Gultyai, T. Ya. Rubinskaya, and A. S. Mendkovich,Izv. Akad. Nauk SSSR, Ser. Khim., 1991, 433 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1991,40, 372 (Engl. Transl.)].
V. P. Gultyai, T. Ya. Rubinskaya, A. S. Mendkovich, and A. I. Rusakov,Izv. Akad. Nauk SSSR, Ser. Khim., 1987, 2812 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1987,36, 2609 (Engl. Transl.)].
A. S. Mendkovich, A. I. Rusakov, G. S. Mironov, and V. P. Gultyai,Izv. Akad. Nauk SSSR, Ser. Khim., 1987, 106 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1987,36, 90 (Engl. Transl.)].
A. S. Mendkovich,Elektrokhimiya, 1992,28, 485 [Russ. Electrochem., 1992,28 (Engl. Transl.)].
A. S. Mendkovich, L. V. Mikhalchenko, and V. P. Gultyai,J. Electroanal. Chem., 1987,224, 273.
V. P. Gultyai, N. K. Lisitsina, A. V. Ignatenko, and A. S. Mendkovich,Izv. Akad. Nauk SSSR, Ser. Khim., 1991, 873 [Bull. Acad. Sci. USSR, Div. Chem. Sci., 1991,40, 771 (Engl. Transl.)].
M. E. Kuchne and B. F. Lambert,Org. Synth., 1963,43, C22.
Author information
Authors and Affiliations
Additional information
Translated fromIzvestiya Akademii Nauk. Seriya Khimicheskaya, No. 10, pp. 1735–1738, October, 1993.
Rights and permissions
About this article
Cite this article
Rubinskaya, T.Y., Mendkovich, A.S., Lisitsina, N.K. et al. The dimerization of radical anions of aromatic carboxylic acids competing with the self-protonation reaction. Russ Chem Bull 42, 1658–1661 (1993). https://doi.org/10.1007/BF00697034
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00697034