Summary
The Stomatogastric ganglion ofPanulirus interruptus contains about 30 neurons, and controls the movements of the lobster's stomach. When experimentally isolated, the ganglion continues to generate complex rhythmic patterns of activity in its motor neurons which are similar to those seen in intact animals.
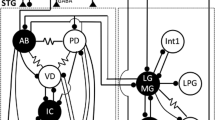
In this paper, we describe the synaptic organization of a group of six neurons which drive the stomach's lateral teeth (Figs. 2, 6). This group includes four motor neurons and two interneurons, all but one of which were recorded and stimulated with intracellular microelectrodes.
One pair of synergistic motor neurons, LGN and MGN, are electrotonically coupled and reciprocally inhibitory (Figs. 9, 12). A second pair of synergistic motor neurons, the LPGNs, are antagonists of LGN and MGN. The LPGNs are electrotonically coupled (Fig. 14), and are both inhibited by LGN and MGN (Figs. 8, 11). The LPGNs inhibit MGN (Fig. 15) but not LGN. One of the two interneurons in the ganglion, Int 1, reciprocally inhibits both LGN and MGN (Figs. 10, 13). The other interneuron, Int 2, excites Int 1 and inhibits the LPGNs (Fig. 16). The synaptic connections observed in the ganglion are reflected in the spontaneous activity recorded from the isolated ganglion and from intact animals.
From the known synaptic organization and observations on the physiological properties of each of the neurons, we have formulated some hypotheses about the pattern-generating mechanism. We found no evidence that any of the neurons are endogenous bursters.
Similar content being viewed by others
References
Alkon, D. L., Fuortes, M. G. F.: Responses of photoreceptors inHermissenda. J. gen. Physiol.60, 631–649 (1972)
Arvanitaki, A., Chalazonites, N.: “Electrical properties and temporal organization in oscillatory neurons”. In: Symp. on Neurobiol. of Invertebrates, p. 169–199, ed. J. Salanki. New York: Plenum 1968
Bullock, T. H., Horridge, G. A.: Structure and function in the nervous systems of invertebrates, 2 vols. San Francisco: Freeman 1965
Dando, M. R., Selverston, A. I.: Command fibers from the supraoesophageal ganglion to the stomatogastric ganglion inPanulirus argus. J. comp. Physiol.78, 138–175 (1972)
Delong, M.: Central patterning of movement. Neurosci. Res. Prog. Bull.9, 10–30 (1971)
Dennis, M. J.: Electrophysiology of the visual system in a Nudibranch mollusc. J. Neurophysiol.30, 1439–1465 (1967)
Huxley, T. H.: The crayfish: an introduction to the study of zoology. London: Kegan Paul & Co 1880
Kater, S. B., Kaneko, C. R. S.: An endogenously bursting neuron in the gastropod,Helisoma trivolvis: Characterization of activityin vivo. J. comp. Physiol.79, 1–14 (1972)
Larimer, J. L., Kennedy, D.: Visceral afferent signals in the crayfish stomatogastric ganglion. J. exp. Biol.44, 345–354 (1966)
Maynard, D. M.: Simpler networks. Ann. N. Y. Acad. Sci.193, 59–72 (1972)
Maynard, D. M., Atwood, H. A.: Divergent postsynaptic effects produced by single motor neurons of the lobster stomatogastric ganglion. Amer. Zool.9, 248 (abstract) (1969)
Maynard, D. M., Dando, M. R.: The structure of the stomatogastric neuromuscular system inPanulirus argus, Homarus americanus, andCallinectes sapidus. J. comp. Physiol. (in press)
Morris, J., Maynard, D. M.: Recordings from the stomatogastric nervous system in intact lobster. Comp. Biochem. Physiol.33, 969–974 (1970)
Mulloney, B.: Organization of flight motoneurons of Diptera. J. Neurophysiol.33, 86–95 (1970)
Mulloney, B., Selverston, A.: Antidromic action potentials fail to demonstrate known interactions between neurons. Science177, 69–72 (1972).
Mulloney, B., Selverston, A. I.: Organization of the stomatogastric ganglion of the spinyl obster. III. Coordination of the two Subsets of the Gastric System. J. comp. Physiol.91, 53–78 (1974)
Orlov, J.: Das Magenganglion des Flußkrebses. Z. mikr.-anat. Forsch.8, 73–96 (1927)
Orlov, J.: Über den histologischen Bau der Ganglien des Mundmagennerven-systems der Crustaceen. Z. Zellforsch.8, 493–541 (1928/9)
Parker, T. J.: On the stomach of the freshwater crayfish. J. Anat. Physiol. (quoted in Huxley, 1880) (1876)
Pearson, K. G., Stein, R. B., Malhotra, S. K.: Properties of action potentials from insect motor nerve fibers. J. exp. Biol.53, 299–316 (1970)
Ryall, R. W., Piercy, M. F., Polosa, C.: Intersegmental and intrasegmental distribution of mutual inhibition of Renshaw cells. J. Neurophysiol.34, 700–707 (1971)
Selverston, A. I.: The use of intracellular dye injections in the study of small neural networks. In: Kater and Nicholson, Intracellular staining techniques in neurobiology. Berlin-Heidelberg-New York: Springer 1973
Selverston, A. I., Mulloney, B.: Organization of the stomatogastric ganglion of the spiny lobster. II. Neurons driving the medial tooth. J. comp. Physiol.91, 33–51 (1974)
Spira, M. E., Bennett, M. V. L.: Synaptic control of electrotonic coupling between neurons. Brain Res.37, 294–300 (1972)
Stein, P. S. G.: Intersegmental coordination of swimmeret motoneuron activity in the crayfish. J. Neurophysiol.34, 310–318 (1971)
Wilson, D. M.: Central nervous mechanisms for the generation of rhythmic behavior in arthropods. Symp. Soc. exp. Biol.20, 199–228 (1966)
Wilson, D. M., Wyman, R. J.: Motor output patterns during random and rhythmic stimulation of locust thoracic ganglia. Biophys. J.5, 121–143 (1965)
Wyman, R. J.: Multi-stable firing patterns among several neurons. J. Neurophysiol.29, 807–833 (1966)
Younge, C. M.: Studies on the comparative physiology of digestion. 2. The mechanism of feeding, digestion, and assimilation inNephrops norvegicus. J. exp. Biol.1, 343–389 (1924)
Author information
Authors and Affiliations
Additional information
We thank D. Kennedy, Eve Marder, and D. Russell for criticizing early drafts of these papers, Nina Pollack and Betty Jorgensen for expert technical assistance, Diane Newsome, SanDee Newcomb, and Pattie Macpherson for typing the many drafts. The authors' research is supported by grant number NS-09322 from N.I.H. and by the Alfred P. Sloan Foundation. B. M. is an NINDS-NIH postdoctoral fellow.
Rights and permissions
About this article
Cite this article
Mulloney, B., Selverston, A.I. Organization of the stomatogastric ganglion of the spiny lobster. J. Comp. Physiol. 91, 1–32 (1974). https://doi.org/10.1007/BF00696154
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00696154