Abstract
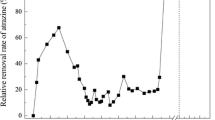
Nitrobenzene was completely degraded by mixed cultures using a sequential anaerobic-aerobic treatment process. Under anaerobic conditions in a fixed-bed column aniline was formed from nitrobenzene through gratuitous reduction by cells of sewage sludge. This reaction was accelerated by the addition of glucose. Complete mineralization of aniline was accomplished by subsequent aerobic treatment using activated sludge as inoculum. The maximum degradation rate of nitrobenzene (4.5 mM) in the two-stage system was 552 mg l−1d−1, referring to 154 mg of nitrobenzene per gram of glucose. In a second experimental phase glucose as cosubstrate and H-donor was replaced by synthetic waste containing ethanol, methanol, isopropanol and acetone. Again, nitrobenzene (1.9 mM) was completely degraded (maximum degradation rate of 237 mg l−d−1, referring to 251 mg per gram of solvents). The major advantage of the described two-stage process is that the reduction of nitrobenzene by anaerobic pretreatment drastically reduces emission by stripping during aerobic treatment.
Similar content being viewed by others
Abbreviations
- HRT:
-
hydraulic retention time
- OD546 :
-
optical density at 546 nm
References
Bergmeyer HU, Bernt U, Schmidt F & Stork H (1974) D-Glucose: Determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU (Ed) Methods of Enzymatic Analysis, 2nd edn (pp 1196–1201). Verlag Chemie, Weinheim, Germany and Academic Press, New York, USA
Beunik J & Rehm H-J (1990) Coupled reductive and oxidative degradation of 4-chloro-2-nitrophenol by a co-immobilized mixed culture system. Appl. Microbiol. Biotechnol. 34: 108–115
Beutler H-O (1984) Ethanol. In: Bergmeyer HU (Ed) Methods of Enzymatic Analysis, 3d edn, Vol. VI (pp 598–606) Verlag Chemie, Weinheim, Germany and Academic Press, New York, USA
Bradford MM (1976) A rapid and sensitive method for the quantitation of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254
Bruhn C, Lenke H & Knackmuss H-J (1987) Nitrosubstituted aromatic compounds as nitrogen source for bacteria. Appl. Environ. Microbiol. 53: 208–210
Dickel O & Knackmuss H-J (1991) Catabolism of 1,3-dinitrobenzene byRhodococcus sp. QT-1. Arch. Microbiol. 157: 76–79
Dorn E & Knackmuss H-J (1978a) Chemical structure and biodegradability of halogenated aromatic compounds: two catechol 1,2-dioxygenases from a 3-chlorobenzoate grownPseudomonad. Biochem. J. 174: 73–84
—— (1978b) Chemical structure and biodegradability of halogenated aromatic compounds: substituent effects on 1,2-dioxygenation of catechol. Biochem. J. 174: 85–94.
Gomolka E & Gomolka B (1979) Ability of activated sludge to degrade nitrobenzene in municipal wastewater. Acta Hydrochim. Hydrobiol. 7: 605–622
Haas R & von Löw E (1986) Grundwasserbelastung durch eine Altlast. Die Folgen einer ehemaligen Sprengstoffproduktion für die heutige Trinkwassergewinnung. Forum Städte-Hygiene 37: 33–43
Haigler BE & Spain JC (1991) Biotransformation of nitrobenzene by bacteria containing toluene degradative pathways. Appl. Environ. Microbiol. 57: 3156–3162
Hallas LE & Alexander M (1983) Microbial transformation of nitroaromatic compounds in sewage effluent. Appl. Environ. Microbiol. 45: 1234–1241
Haug W, Schmidt A, Nörtemann B, Hempel DC, Stolz A & Knackmuss H-J (1991) Mineralization of the sulfonated azo dye Mordant Yellow 3 by a 6-aminonaphthalene-2-sulfonate-degrading bacterial consortium. Appl. Environ. Microbiol. 57: 3144–3149
Leisinger, T & Brunner W (1986) Poorly degradable substances. In: Rehm H-J & Reed G (Eds) Biotechnology, Vol. 8 (pp 475–513). Verlag Chemie, Weinheim, Germany
Lenenberger C, Czuczwa J, Tremp J & Giger W (1988) Nitrated phenols in rain: Atmospheric occurrence of phytotoxic pollutants. Chemosphere 17: 511–515
Nakazawa T & Yokota T (1973) Benzoate metabolism inPseudomonas putida (arvilla) mt-2: demonstration of two benzoate pathways. J. Bacteriol. 115: 262–267
Nishino SF & Spain J (1993) Degradation of nitrobenzene by aPseudomonas speudoalcaligenes. Appl. Environ. Microbiol. 59: 2520–2525.
Nozaki M (1970) Metapyrocatechase (Pseudomonas). In: Tabor H & Tabor CW (Eds) Methods in Enzymology, Vol 17A (pp 522–525). Academic Press, New York, USA
Patil SS & Shinde VM (1988) Biodegradation studies of aniline and nitrobenzene in aniline plant wastewater by gas chromatography. Environ. Sci. Technol. 22: 1160–1165
Patil SS & Shinde VM (1989) Gas chromatographic studies on the biodegradation of nitrobenzene and 2,4-dinitrophenol in the nitrobenzene plant wastewater. Environ. Poll. 57: 235–250
Pitter P (1976) Determination of biological degradability of organic substances. Water Res. 10: 231–235
Rippen G, Zietz E, Frank R, Knacker T & Klöpffer W (1987) Do airborne nitrophenols contribute to forest decline? Environ. Technol. Lett. 8: 475–482
Schmidt E & Schmidt FW (1983) Glutamate Dehydrogenase. In: Bergmeyer HU (Ed) Methods of Enzymatic Analysis, 3d edn, Vol III (pp 216–227). Verlag Chemie, Weinheim, Germany and Academic Press, New York, USA
Schmidt E, Hellwig M & Knackmuss H-J (1983) Degradation of chlorophenols by a defined mixed microbial community. Appl. Environ. Microbiol. 36: 1038–1044
Schmidt K, Liaaen Jensen S & Schlegel HG (1963) Die Carotinoide der Thiorhodaceae. I Okenon als Hauptcarotinoid vonChromatium okenii Perty. Arch. Mikrobiol. 46: 117–126
Sedmak JJ & Grossberg SE (1977) A rapid, sensitive and versatile assay for protein using Coomassie Brilliant Blue G 250. Anal. Biochem. 79: 544–552
Spanggord RJ, Spain JC, Nishino SF & Mortelmans KE (1991) Biodegradation of 2,4-dinitrotoluene by aPseudomonas sp. Appl. Environ. Microbiol. 57: 3200–3205
Wirth W, Hecht G & Gloxhuber C (1971) Toxikologie-Fiebel (pp 264–266). Georg Thieme Verlag, Stuttgart, Germany
Zoeteman BCJ, Harmsen K, Linders JBHJ, Morra CFH & Slooff M (1980) Persistent organic pollutants in river water and ground water of the Netherlands. Chemosphere 9: 231–249
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dickel, O., Haug, W. & Knackmuss, HJ. Biodegradation of nitrobenzene by a sequential anaerobic-aerobic process. Biodegradation 4, 187–194 (1993). https://doi.org/10.1007/BF00695121
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00695121