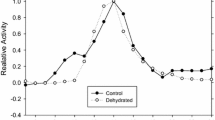
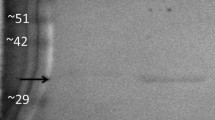
Summary
The regulatory properties of type L pyruvate kinase fromRana pipiens are intermediate between those of the mammalian K and L isozymes. As with mammalian type L, the levels of the frog isozyme are affected by the animal's nutritional state. The mammalian and amphibian isozymes show similar sensitivities to fructose 1,6-bisphosphate activation and amino acid inhibition. By contrast, the frog L isozyme shares several properties of the K class: ie. irreversible inactivation by oxidized glutathione and lack of response to a cyclic AMP stimulated phosphorylation. Furthermore, as for some mammalian K isozymes, frog type L shows a high PEP affinity and a low cooperativity of PEP binding.
Insofar as the properties of this present day enzyme reflect those of its counterpart in the amphibian ancestor of higher vertebrates, our results suggest that at its first expression, the type L resembled the type K. Many important regulatory properties of the L isozyme, especially the sensitivity to phosphorylation, were acquired more recently perhaps in association with an increased importance of constant blood glucose.
Similar content being viewed by others
Abbreviations
- DTT :
-
dithiothreitol
- EDTA :
-
ethylenediamine tetraacetic acid
- EGTA :
-
ethyleneglycol-bis(2-aminoethylether)-N,N′-tetraacetic acid
- FB :
-
fructose-1,6-bisphosphate
- PEP :
-
phosphoenolpyruvate
- PK :
-
pyruvate kinase
References
Bell DJ (1971) Plasma glucose. In: Bell DJ, Freeman BM (eds) Physiology and biochemistry of the domestic fowl, vol II. Academic Press, New York, pp 913–919
Brinn JE Jr (1978) The pancreatic islets of bony fishes. Am Zool 13:653–665
Carbonell J, Feliu JE, Marco R, Sols A (1973) Pyruvate kinase: classess of regulatory isoenzymes in mammalian tissues. Eur J Biochem 37:148–156
Cardenas JM, Dyson RD (1973) Bovine pyruvate kinase. II. Purification of the liver isozyme and its hybridization with skeletal muscle pyruvate kinase. J Biol Chem 248:6938–6944
Cardenas JM, Blachly EC, Ceccotti PL, Dyson RD (1975a) Properties of chicken skeletal muscle pyruvate kinase and a proposal for its evolutionary relationship to the other avian and mamalian isozymes. Biochemistry 14:2247–2252
Cardenas JM, Strandholm, JJ, Miller JM (1975b) Effects of phenylalanine and alanine on the kinetics of bovine pyruvate kinase isozymes. Biochemistry 14:4041–4045
Carneiro NM, Amaral AD (1983) Effects of insulin and glucagon on plasma glucose levels and glycogen content in organs of the freshwater teleostPimelodus maculatus. Gen Comp Endocrinol 49:115–121
Claus TH, El-Maghrabi MR, Pilkis SJ (1979) Modulation of the phosphorylation state of rat liver pyruvate kinase by allosteric effectors and insulin. J Biol Chem 254:7855–7864
Eigenbrodt E, Schoner W (1977) Purification, and properties of the pyruvate kinase isoenzymes type L and M2 from chicken liver. Hoppe-Seyler's Z Physiol Chem 358:1033–1046
El-Maghrabi MR, Haston WS, Flockhart DA, Claus, T, Pilkis SJ (1980) Studies on the phosphorylation and dephosphorylation of L-type pyruvate kinase by the catalytic subunit of cyclic AMP-dependent protein kinase. J Biol Chem 255:668–675
Farrar ES, Frye BE (1979) Factors affecting normal carbohydrate levels inRana pipiens. Gen Comp Endocrinol 39:358–371
Flanders LE, Bamburg JR, Sallach HJ (1971) Pyruvate kinase isozymes in adult tissue and eggs ofRana pipiens. Biochim Biophys Acta 242:566–579
Geelen MJH, Harris RA, Beynen AC, McCune SA (1980) Short-term hormonal control of hepatic lipogenesis. Diabetes 29:1006–1022
Gilbert HF (1982) Biological disulfides: the third messenger? J Biol Chem 257:12086–12091
Guderley HE, Cardenas JM (1979) Developmental changes in the pyruvate kinase isozymes of coho salmon J Exp Zool 208:1–12
Guderley HE, Hamel L, Lafond J (1983) Close resemblance between muscle pyruvate kinase from a primitive vertebrate, the river sturgeonAcipenser fulvenscens, and the ancestral type K isozyme. J Comp Physiol 153:247–256
Hall ER, Cottam GL (1978) Isozymes of pyruvate kinase in vertebrates: their physical, chemical and immunological properties. Int J Biochem 9:785–793
Hers HG, Hue L (1983) Gluconeogenesis and related aspects of glycolysis. Annu Rev Biochem 52:617–653
Humble E, Berglund L, Titanji V, Ljungström O, Edlund B, Zetterqvist Ö, Engström L (1975) Non-dependence on native structure of pig liver pyruvate kinase when used as a substrate for cyclic 3′5′AMP stimulated protein kinase. Biochem Biophys Res Commun 66:614–621
Ibsen KH, Murray L, Marles SW (1976) Electrofocusing and kinetic studies of adult and embryonic chicken pyruvate kinases. Biochemistry 15:1064–1073
Ibsen KH, Cardin JP Jr, Chiu BH-C, Garratt KN, Marles SW, Doty JR (1980) Distribution of pyruvate kinase isozymes in adult and developingXenopus laevis. Comp Biochem Physiol 65B:473–480
Imamura K, Tanaka T (1972) Multimolecular forms of pyruvate kinase from rat and other mammalian tissues. J Biochem 71:1043–1051
Imamura K, Taniuchi K, Tanaka T (1972) Multimolecular forms of pyruvate kinase. J Biochem 72:1001–1015
Imamura K, Tanaka T, Nishina T, Nakashima K, Miwa S (1973) Studies on pyruvate kinase deficiency. J Biochem 74:1165–1175
Irving MG, Williams JF (1973) Kinetic studies on the regulation of rabbit liver pyruvate kinase. Biochem J 131:287–301
Jensen RA, Nester EW (1966) Regulatory enzymes of aromatic amino acid biosynthesis inBacillus subtilis. J Biol Chem 241:3373–3380
Kohl EA, Cottam GL (1976) Alteration in liver pyruvate kinase protein and catalytic activity upon starvation and refeeding. Arch Biochem Biophys 176:671–682
Krebs HA, Eggleston LW (1965) The role of pyruvate kinase in the regulation of gluconeogenesis Biochem J 94:3c
Kutzbach, C, Bischofberger H, Hess B, Zimmermann-Telschow H (1973) Pyruvate kinase from pig liver. Hoppe-Seyler's Z Physiol Chem 354:1473–1489
Ljungström O, Helmquist G, Engström L (1974) Phosphorylation of purified rat liver pyruvate kinase by cyclic AMP-stimulated protein kinase. Biochem Biophys Acta 358:289–298
Marco R, Carbonell J, Llorente P (1971) Allosteric properties of adipose tissue pyruvate kinase. Biochem Biophys Res Commun 43:126–132
Peters J, Nash HR, Eicher EM, Bulfield G (1981) Polymorphism of kidney pyruvate kinase in the mouse is determined by a gene, PK-3, on chromosome 9. Biochem Gen 19:757–769
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:346–356
Rigaut J-P, Chalumeau M-T (1984) Pyruvate kinase isozyme patterns of fish. Comp Biochem Physiol 77B:451–458
Romsos DR, Leveille GA (1974) Effect of diet on activity of enzymes involved in fatty acid and cholesterol synthesis. Adv Lip Res 12:97–146
Roos R de, Parker AV (1982) Nondetectable plasma glucose levels after insulin administration in the American bullfrog (Rana catesbeiana) Gen Comp Endocrinol 46:505–510
Saheki S, Saheki K, Tanaka T (1982a) Peptide structures of pyruvate kinase isozymes. 1. Comparison of the four pyruvate kinase isozymes of the rat. Biochim Biophys Acta 704:484–493
Saheki S, Saheki K, Tanaka T, Tanaka T (1982b) Peptide structures of pyruvate kinase isozymes. 2. Origins of types M1 and M2 isozymes suggested from species-variations in their peptide maps. Biochim Biophys Acta 704:494–502
Schloen LH, Kmiotek EH, Sallach HJ (1974) Pyruvate kinase isozymes in adult tissues and eggs ofRana pipiens. Arch Biochem Biophys 164:254–265
Strandholm JJ, Cardenas JM, Dyson RD (1975) Pyruvate kinase isozymes in adult and fetal tissues of chicken. Biochemistry 14:2242–2246
Van Berkel ThJC (1974) Some kinetic properties of M2-type pyruvate kinase from rat liver at physiological Mg++ concentration. Biochim Biophys Acta 370:140–152
Van Berkel ThJC, Koster JF (1973) M-type pyruvate kinase of leucocytes: an allosteric enzyme. Biochim Biophys Acta 293:134–139
Van Berkel ThJC, Koster JC, Hülsmann WC (1973a) Two interconvertible forms of L-type pyruvate kinase from rat liver. Biochim Biophys Acta 293:118–124
Van Berkel ThJC, Koster JF, Hülsmann WC (1973b) Some kinetic properties of the allosteric M-type pyruvate kinase from rat liver: influence of pH and the nature of amino acid inhibition. Biochim. Biophys Acta 321:171–180
Van Berkel ThJC, Kruijt JK, van den Berg GB, Koster JF (1978) Difference in the effect of glucagon and starvation upon L-type pyruvate kinase from rat liver. Eur J Biochem 92:553–561
Van den Berg GB, Van Berkel ThJC, Koster JF (1978) Cyclic AMP-dependent inactivation of human liver pyruvate kinase. Biochem Biophys Res Commun 82:859–864
Walton MJ, Cowey CB (1982) Aspects of intermediary, metabolism in salmonid fish. Comp Biochem Physiol 83B:59–79
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fournier, P., Guderley, H. Evolution of the functional properties of pyruvate kinase isozymes: pyruvate kinase L fromRana pipiens . J Comp Physiol B 156, 691–699 (1986). https://doi.org/10.1007/BF00692747
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00692747